Application of teprenone in prevention and treatment of morphine-induced liver injury
A technology for teprenone and liver damage, applied in the field of medicine, to achieve high safety, reduce economic burden, and significant curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
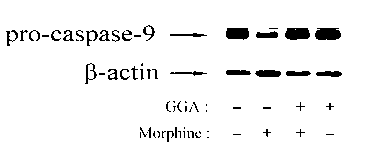
[0024] Example 1: Teprenone inhibits morphine-induced activation of caspase-9 in the liver
[0025] Mice were divided into 4 groups (6 mice in each group): control group (saline), morphine group (Mor), GGA and morphine group (GGA+Mor), and GGA group. From day 1 to day 7, Saline group and Mor group were pre-administered with normal saline, GGA+Mor group and GGA group were pre-administered with GGA (800 mg / kg), 2 hours later, Saline group and GGA group were intraperitoneally injected Normal saline, Mor group and GGA+Mor group received intraperitoneal injection of morphine (daily injection dose: 10, 20, 40, 60, 80, 100 and 100 mg / kg). Two hours after the last morphine injection, the liver tissue of the mice was isolated, the protein was extracted, and the expression of pro-caspase-9 in the liver cells was detected by Western blotting. figure 1 The results showed that pre-infusion of GGA could inhibit the reduction of pro-caspase-9 induced by morphine, which indicated that tepr...
Embodiment 2
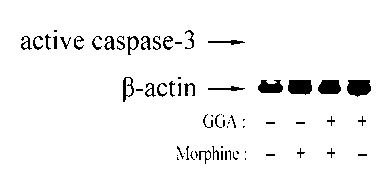
[0026] Example 2: Teprenone inhibits morphine-induced activation of caspase-3 in the liver
[0027] Mice were divided into 4 groups (6 mice in each group): control group (saline), morphine group (Mor), GGA and morphine group (GGA+Mor), and GGA group. From day 1 to day 7, Saline group and Mor group were pre-administered with normal saline, GGA+Mor group and GGA group were pre-administered with GGA (800 mg / kg), 2 hours later, Saline group and GGA group were injected intraperitoneally Normal saline, Mor group and GGA+Mor group received intraperitoneal injection of morphine (daily injection dose: 10, 20, 40, 60, 80, 100 and 100 mg / kg). Two hours after the last morphine injection, the liver tissue of the mice was isolated, the protein was extracted, and the expression of caspase-3 in liver cells was detected by Western blotting. figure 2 The results showed that pre-infusion of GGA could inhibit the increase of active caspase-3 induced by morphine, which indicated that teprenone c...
Embodiment 3
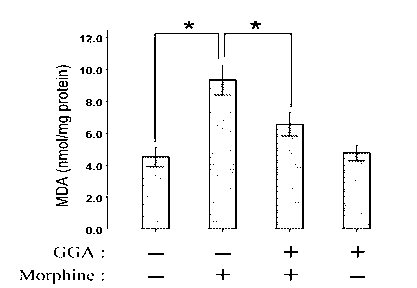
[0029] Example 3: Teprenone inhibits morphine-induced increases in malondialdehyde (MDA) in the liver
[0030] Mice were divided into 4 groups (6 mice in each group): control group (saline), morphine group (Mor), GGA and morphine group (GGA+Mor), and GGA group. From day 1 to day 7, Saline group and Mor group were pre-administered with normal saline, GGA+Mor group and GGA group were pre-administered with GGA (800 mg / kg), 2 hours later, Saline group and GGA group were injected intraperitoneally Normal saline, Mor group and GGA+Mor group received intraperitoneal injection of morphine (daily injection dose: 10, 20, 40, 60, 80, 100 and 100 mg / kg). Two hours after the last morphine injection, the liver tissue of the mice was separated, prepared into a 10% homogenate with phosphate buffer in an ice bath, and centrifuged in a high-speed refrigerated centrifuge for 10 minutes (4°C, 4000 rpm). The supernatant was taken, and the content of malondialdehyde (MDA) was detected using malo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com