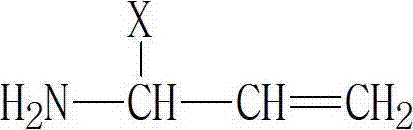Fluoroacrylate pressure sensitive adhesive, and preparation method thereof
An acrylate and pressure-sensitive adhesive technology, applied in the preparation of carboxylic acid amides, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of non-silicon transfer and proposed effective methods, so as to reduce the transfer of silicon, The carbon-fluorine bond can be large, and the effect of improving cohesion and bonding strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The preparation steps of fluorine-containing hard monomer are as follows:
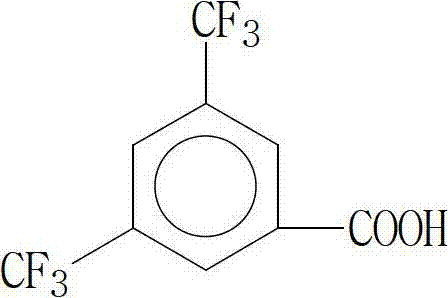
[0053] Dissolve 3,5-bis(trifluoromethyl)benzoic acid in N,N-dimethylformamide, place in a reaction vessel and cool with ice water, add 1-hydroxybenzotriazole and K 2 CO 3 , and then add an equimolar amount of reaction monomer with 3,5-bis(trifluoromethyl)benzoic acid, stir at room temperature for 2~3 hours, add saturated NaHCO 3 Solution reaction, followed by extraction and recrystallization to obtain fluorine-containing hard monomers;
[0054] Among them, 1-hydroxybenzotriazole and K 2 CO 3 The molar mass ratio of 1-hydroxybenzotriazole to 3,5-bis(trifluoromethyl)benzoic acid is 1.5:1;
[0055] The molecular structural formula of the 3,5-bis(trifluoromethyl)benzoic acid is:
[0056]
[0057] The structural characteristics of the described reaction monomer are:
[0058]
[0059] Among them, X is an alkane group containing a hydroxyl group.
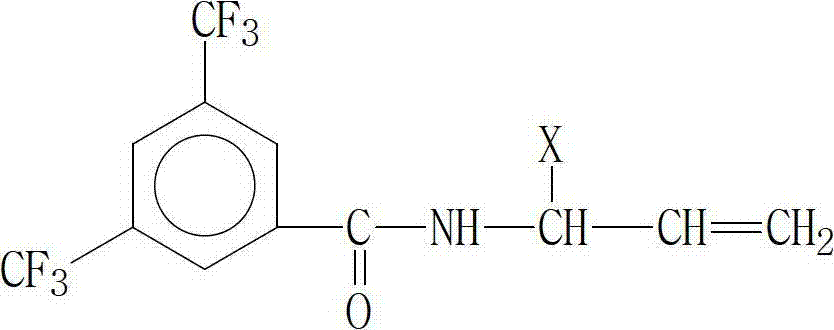
[0060] A preparation method of fluorine-c...
Embodiment 2
[0063] The preparation steps of fluorine-containing hard monomer are as follows:
[0064] Dissolve 3,5-bis(trifluoromethyl)benzoic acid in N,N-dimethylformamide, place in a reaction vessel and cool with ice water, add 1-hydroxybenzotriazole and K 2 CO 3 , and then add an equimolar amount of reaction monomer with 3,5-bis(trifluoromethyl)benzoic acid, stir at room temperature for 2~3 hours, add saturated NaHCO 3 Solution reaction, followed by extraction and recrystallization to obtain fluorine-containing hard monomers;
[0065] Among them, 1-hydroxybenzotriazole and K 2 CO 3 The molar mass ratio of 1-hydroxybenzotriazole to 3,5-bis(trifluoromethyl)benzoic acid is 2:1;
[0066] The molecular structural formula of the 3,5-bis(trifluoromethyl)benzoic acid is:
[0067]
[0068] The structural characteristics of the described reaction monomer are:
[0069]
[0070] Among them, X is an alkane group containing a hydroxyl group.
[0071] A preparation method of fluorine-con...
Embodiment 3
[0074] The preparation steps of fluorine-containing hard monomer are as follows:
[0075] Dissolve 3,5-bis(trifluoromethyl)benzoic acid in N,N-dimethylformamide, place in a reaction vessel and cool with ice water, add 1-hydroxybenzotriazole and K 2 CO 3 , and then add an equimolar amount of reaction monomer with 3,5-bis(trifluoromethyl)benzoic acid, stir at room temperature for 2~3 hours, add saturated NaHCO 3 Solution reaction, followed by extraction and recrystallization to obtain fluorine-containing hard monomers;
[0076] Among them, 1-hydroxybenzotriazole and K 2 CO 3 The molar mass ratio of 1-hydroxybenzotriazole to 3,5-bis(trifluoromethyl)benzoic acid is 1.75:1;
[0077] The molecular structural formula of the 3,5-bis(trifluoromethyl)benzoic acid is:
[0078]
[0079] The structural characteristics of the described reaction monomer are:
[0080]
[0081] Among them, X is an alkane group containing a hydroxyl group.
[0082] A preparation method of fluorine-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com