Method for extracting and separating uranyl ions from water phase containing zirconium ions and lanthanide ions
A technology for separating uranyl ions and uranyl ions, which is applied in the field of nuclear fuel cycle, can solve problems such as difficult separation of zirconium ions, achieve high selective extraction performance, improve extraction capacity, and wide range of aqueous acidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1, extract UO from aqueous phase 2 2+
[0019] When performing extraction experiments, take 0.5mL of ionic liquid containing a certain amount of CMPO and 0.5mL of an aqueous phase containing a certain amount of uranyl ions, zirconium ions (lanthanide ions) and a certain amount of nitric acid, mix thoroughly, then shake for 30min, and centrifuge to separate the phases Then take the upper water phase to analyze the concentration of uranyl ions, zirconium ions, and lanthanide ions (ICP-AES). or distribution ratio D.
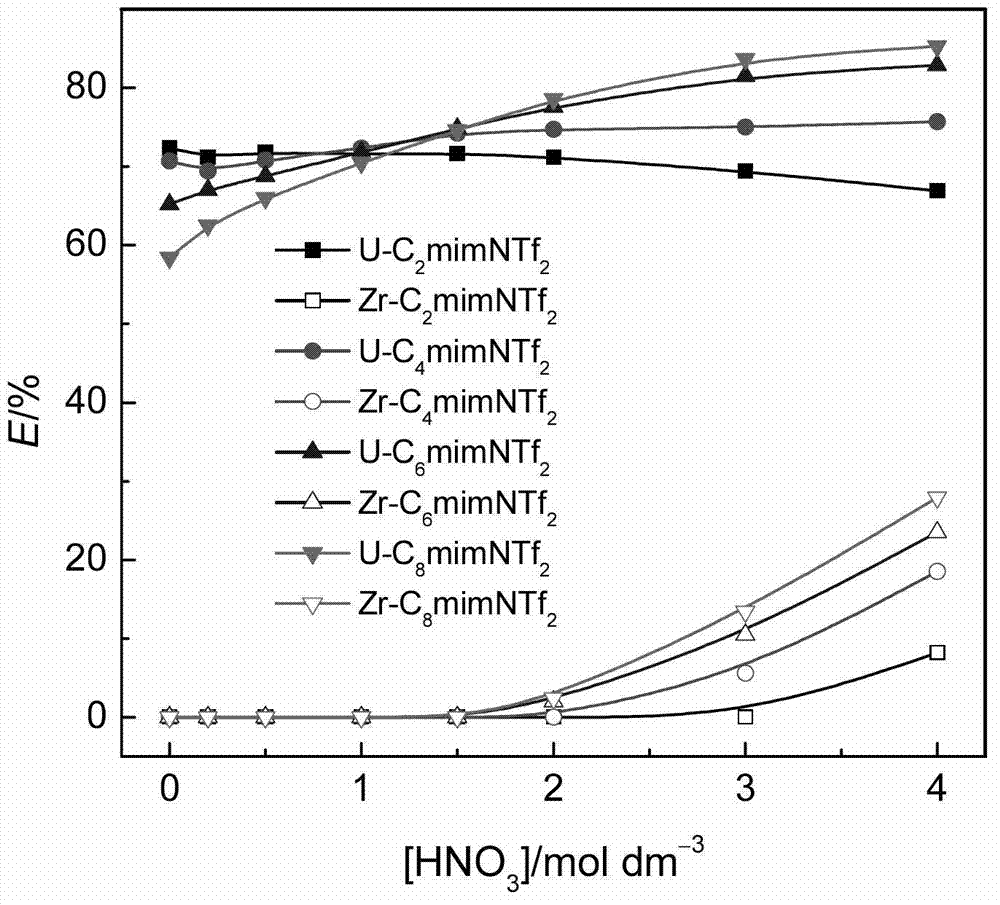
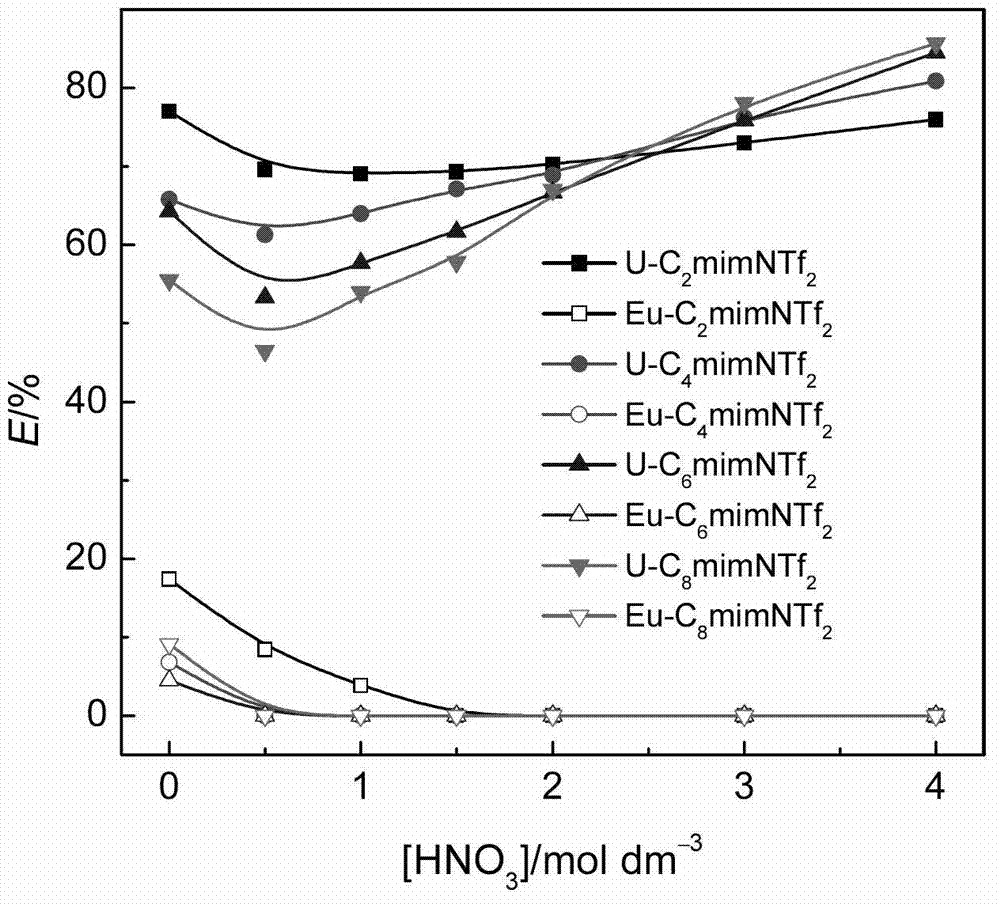
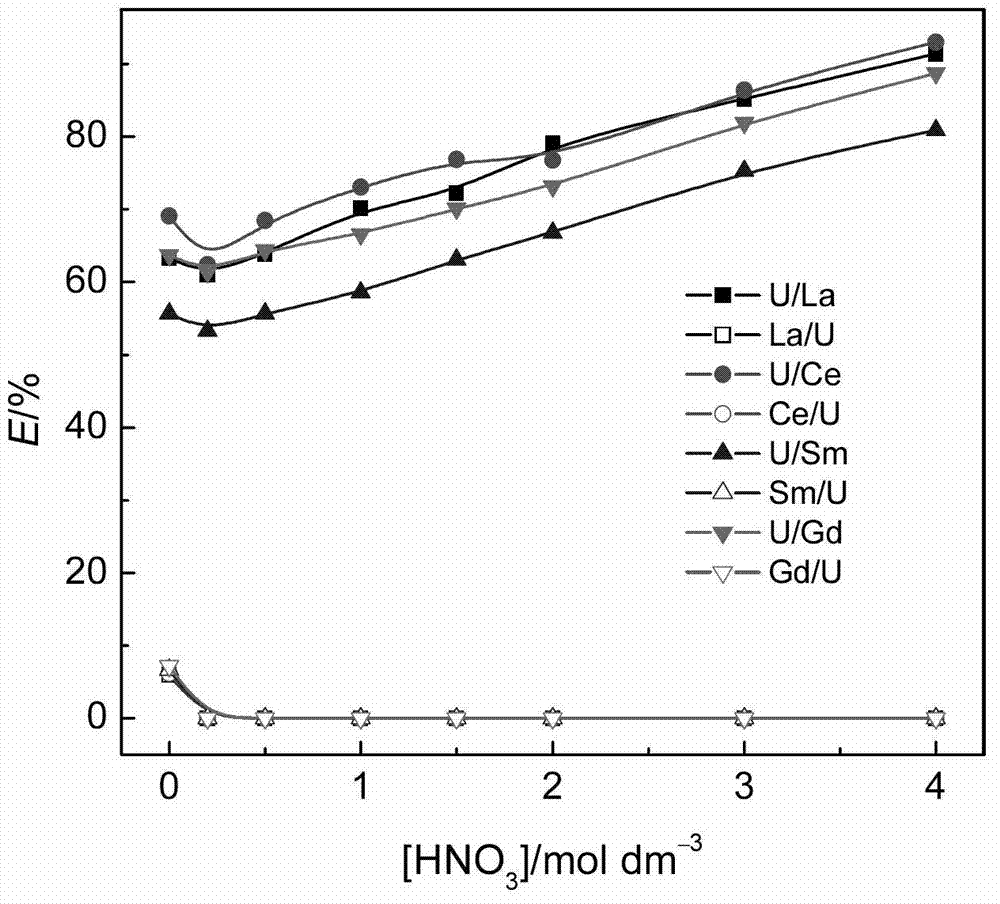
[0020] When different ionic liquids containing 0.02mol / L CMPO extract 0.01mol / L uranyl ions in the presence of 0.01mol / L zirconium ions, the extraction rate varies with the concentration of nitric acid in the aqueous phase, as figure 1 shown. for C 2 mimNTf 2 system, increasing the concentration of nitric acid in the aqueous phase slightly decreased the extraction rate of uranyl ions as a whole. for C 4 mimNTf 2 、C 6 mimNTf 2 、C 8 mimNT...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com