Method for the electrochemical measurement of an analyte concentration in vivo, and fuel cell for this purpose
A fuel cell and analyte technology, which is applied in biochemical fuel cells, biochemical equipment and methods, analytical materials, etc., can solve the problems of removing sensors on fire, and achieve the effect of simple cost and consumption avoidance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0046] figure 1 The sensor shown in works on the principle of a fuel cell. From the analyte to be measured (for example glucose or lactic acid (Lactat)), a redox amphoteric product is produced which forms the fuel for the fuel cell. Thus the greater the analyte concentration to be measured, the greater the energy provided by the fuel cell. The voltage drop across the load resistor between anode and cathode can thus be used as a measurement signal for determining the analyte concentration.
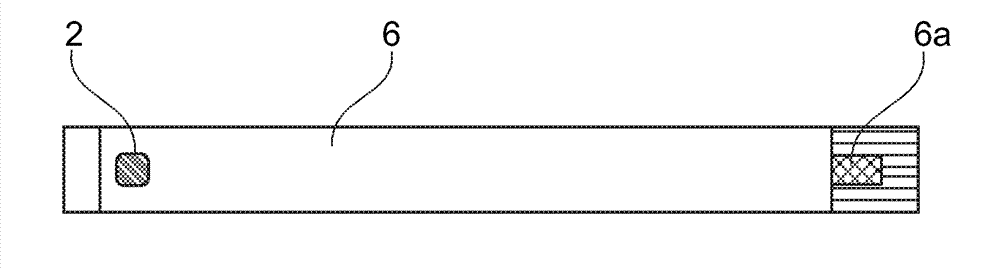
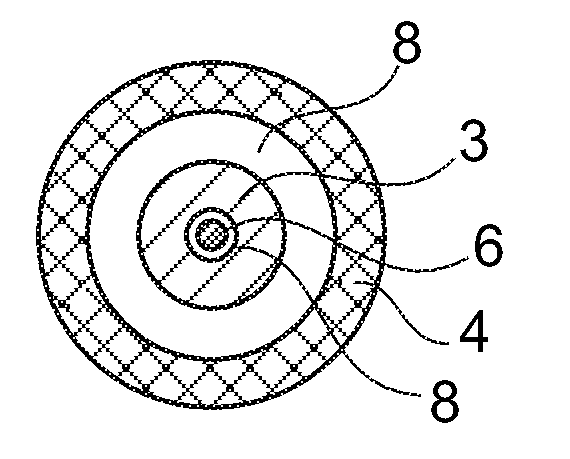
[0047] exist figure 1 in top view and in figure 2 The sensor shown in longitudinal section in has an anode 1 and a cathode 2 which are covered by a common enzyme layer 3 . The anode 1 and the cathode 2 are each located at the end of a conductor track 5 , 6 arranged on a carrier 4 (for example a plastic foil). The conductor tracks 5 , 6 can consist of a noble metal (eg palladium), which can also form the surface of the cathode 2 . The anode 1 can be formed as a coating of the conducto...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com