Modified dendritic cell and vaccine containing the same
A dendritic cell and vaccine technology, which is applied in the fields of biotechnology and medicine, and achieves the effects of simple preparation process and good active immunotherapy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] A modified dendritic cell comprising the SYK gene transfected thereinto using a viral vector. Wherein, the preparation method of the modified dendritic cells is as follows:
[0041] 1. Amplification of SYK gene fragment
[0042] The PCR amplification template is a plasmid (pCMV6-XL5) carrying the full-length cDNA of the SYK gene (NCBI RefSeq: NM 003177.3), which was purchased from OriGen Biological Company. The CDS sequence (SEQ ID NO: 1) of the SYK gene is about 1.9kb.
[0043] The primer sequences used for PCR amplification are:
[0044] P1: 5'-ATGGCCAGCAGCGGCATGGCTG-3' (SEQ ID NO: 2)
[0045] P2: 5'-TTAGTTCACCACGTCATAGTAG-3' (SEQ ID NO: 3)
[0046] Where P1 is the forward primer and P2 is the reverse primer.
[0047] The PCR reaction system for amplifying the SYK gene is as follows:
[0048]
[0049]
[0050] PCR reaction conditions such as Figure 6 shown.
[0051] 2. Purification of SYK gene amplified fragment
[0052] Agarose gel electrophoresis is ...
Embodiment 2
[0106] Determination of DC vaccine and related parameters
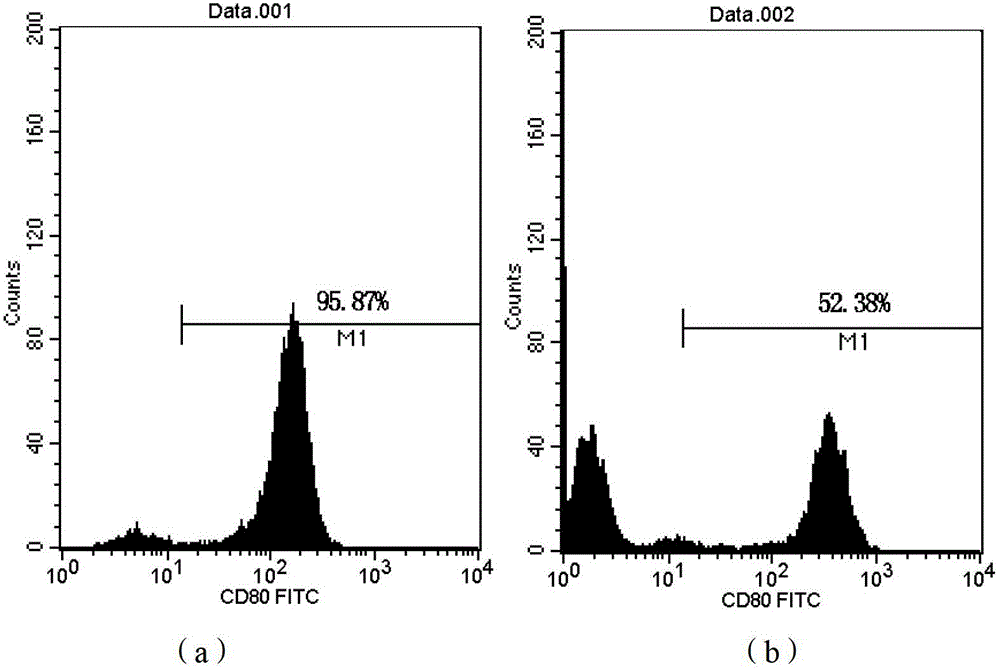
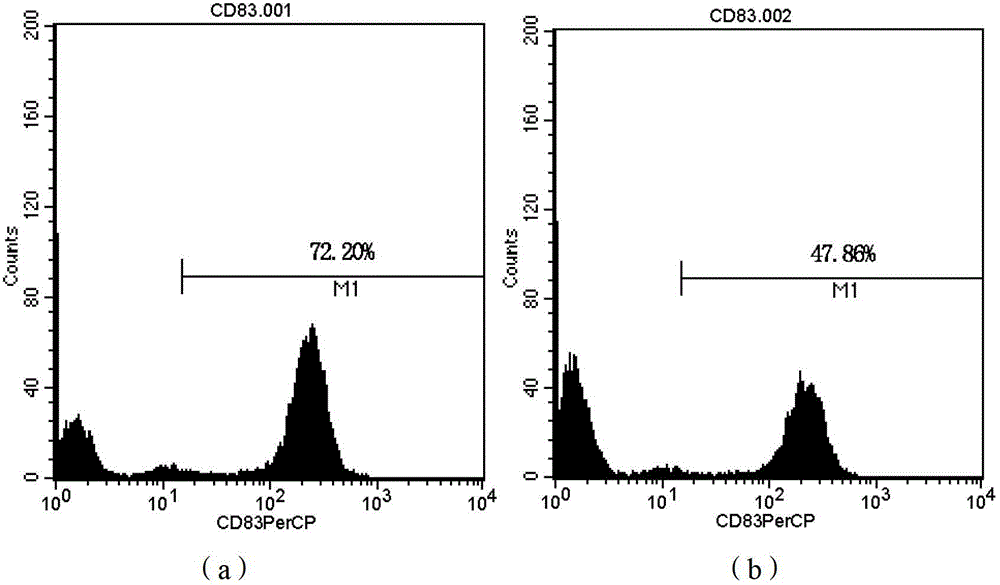
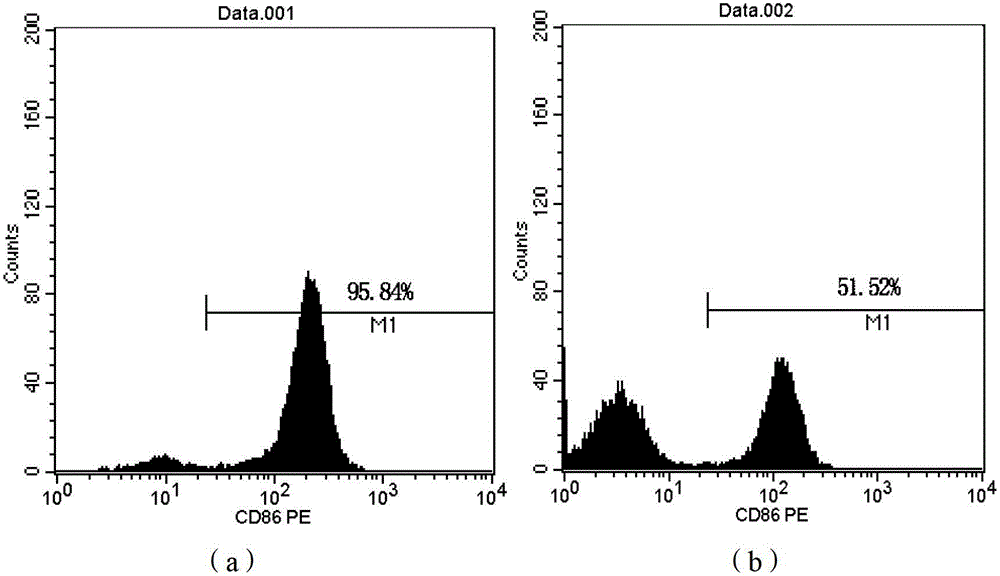
[0107] 1. Detection of DC cell maturity
[0108] The LV-DC cells obtained in Step 8 of Example 1 can be directly used as a DC cell vaccine. The expression of CD80, CD83, and CD86 molecules on the surface of LV-DC cells obtained in Example 1 was detected by flow cytometry, and the detection results are shown in Table 2 and figure 1 , 2 , 3.
[0109] 2. Detection of tumor killing rate of LV-DC-CIK cells in vitro
[0110] Will 2×10 5 The LV-DC cell obtained in step 8 in Example 1 was mixed with 2×10 6 CIK cells obtained in Step 8 were co-cultured (LV-DC:CIK=1:10), supplemented with 0.5% (V / V) autologous plasma, 1000U / ml IL-2, 100ng / ml GM-CSF every two days Alys-505 medium, 5-7 days later to obtain effector cells LV-DC-CIK cells.
[0111] Take CFSE-labeled RB cells as target cells, and adjust the cell density to 1×10 5 / ml and 1×10 6 / ml.
[0112] Take a flow tube, add 800 μl and 200 μl of effector target cells ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com