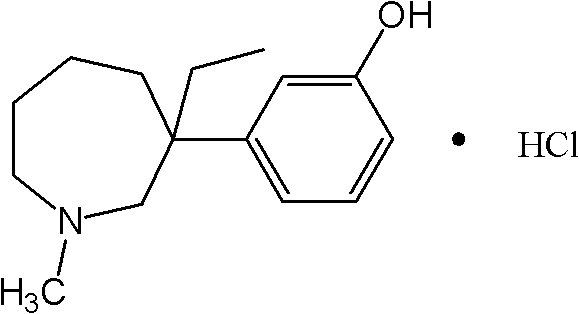
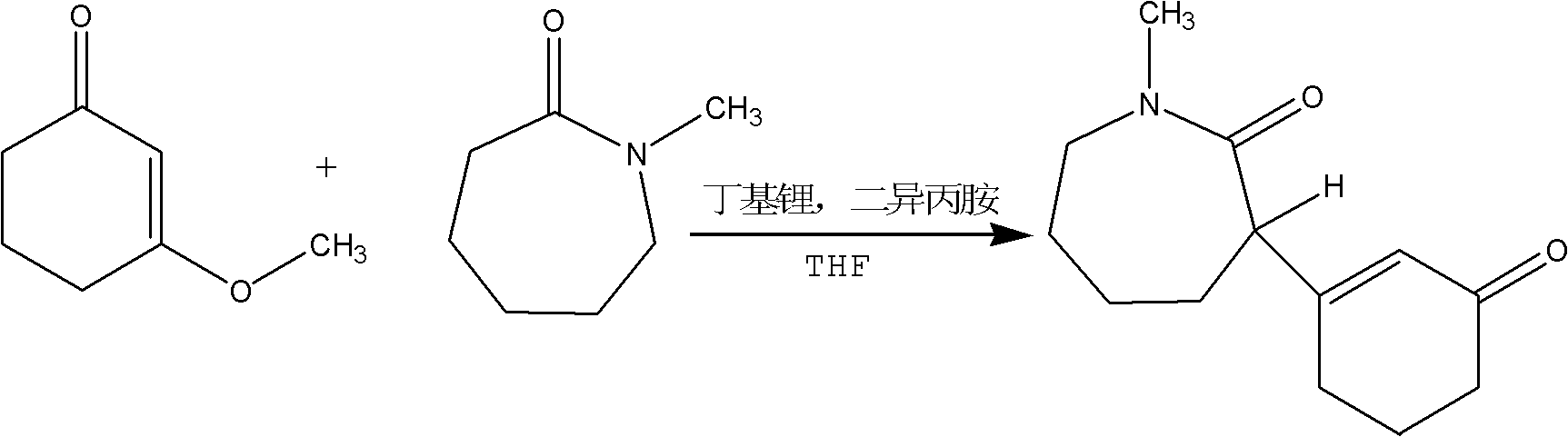
Preparation method for intermediate II during meptazinol hydrochloride synthesis process
A technology of the synthetic process of mebutamol hydrochloride, which is applied in the direction of organic chemistry, etc., to achieve the effects of high yield, shortened time, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0017] Below, in conjunction with embodiment the content of the present invention is described in detail.
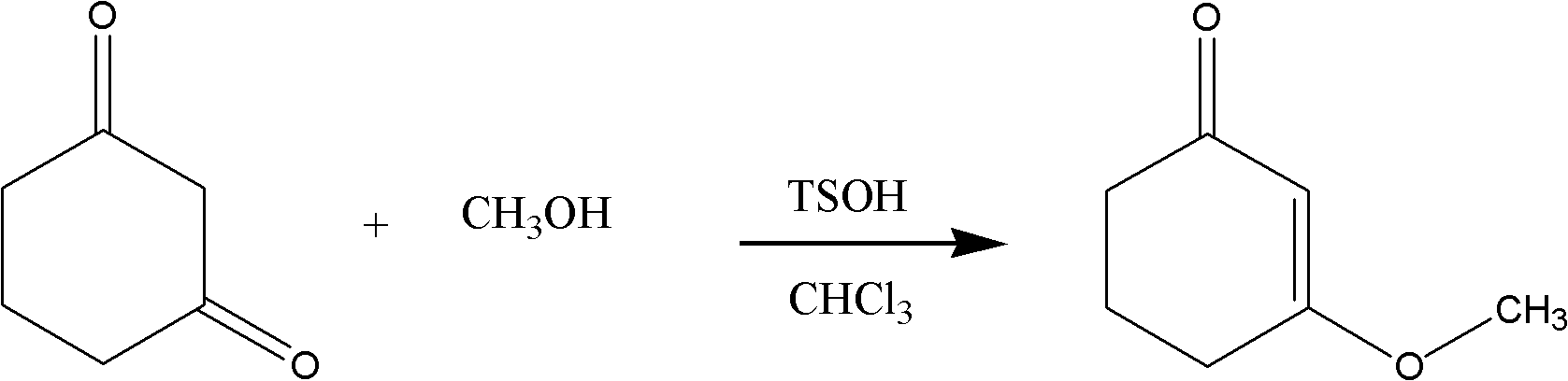
[0018] (1) Preparation of 3-methoxy-2-cyclohexenone (intermediate I)
[0019]
[0020] 1. Feeding ratio:
[0021] 1,3-cyclohexanedione FW=112.05 1800g 16mol
[0022] Methanol FW=32.03 4500g 140mol
[0023] Chloroform d=1.47 24000-28000ml
[0024] 2. Operation steps:
[0025] Add 1,3-cyclohexanedione, chloroform, methanol and 20g of p-toluenesulfonic acid into the reaction flask in sequence, heat up to boiling with stirring, and slowly distill out about 20000ml of distillate, which takes 5 to 7 hours. Remove from heat and cool to room temperature. Samples were taken by TLC to confirm that the reaction was normal and post-processed. Wash the residue with 10000ml of 10% sodium hydroxide for 3 times, combine the lye layers, and extract with 3000ml of distilled liquid. The organic layers were combined, washed with saturated brine 2000ml*2 until neutral, and dried ov...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com