3-Hydroxyprogesterone-21-(2',5'-dimethoxy)benzylidene-5-ene-20-one and preparation method and application thereof
A technology of dimethoxy and benzylidene, which is applied in the field of medicine and can solve unseen problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of the compound 3-hydroxyl-pregna-21-(2′,5′-dimethoxy)benzylidene-5-ene-20-one shown in embodiment 1 structural formula I
[0030]
[0031] Formula II-1 Structural Formula I
[0032] Dissolve 500mg of the compound of formula II-1 in 20ml of methanol, add 1.2 molar equivalents of 2,5-dimethoxybenzaldehyde and 100mg of potassium hydroxide at 0°C, stir for 24h, add 80ml of water, then add 10% HCl Neutralize to pH ~ 6, filter, wash the filtrate with water, and dry to obtain a yellow solid compound represented by structural formula I, with a yield of 90%. 1 H NMR (300MHz, CDCl 3 ): δ7.83(d, 1H), 7.07(d, 1H), 6.92-6.78(m, 3H), 5.34(d, 1H), 3.83(s, 3H), 3.78(s, 3H), 3.52( m, 1H), 0.98(s, 3H), 0.63(s, 3H). ESI-MS: 465.4 [M+H + ].
Embodiment 2
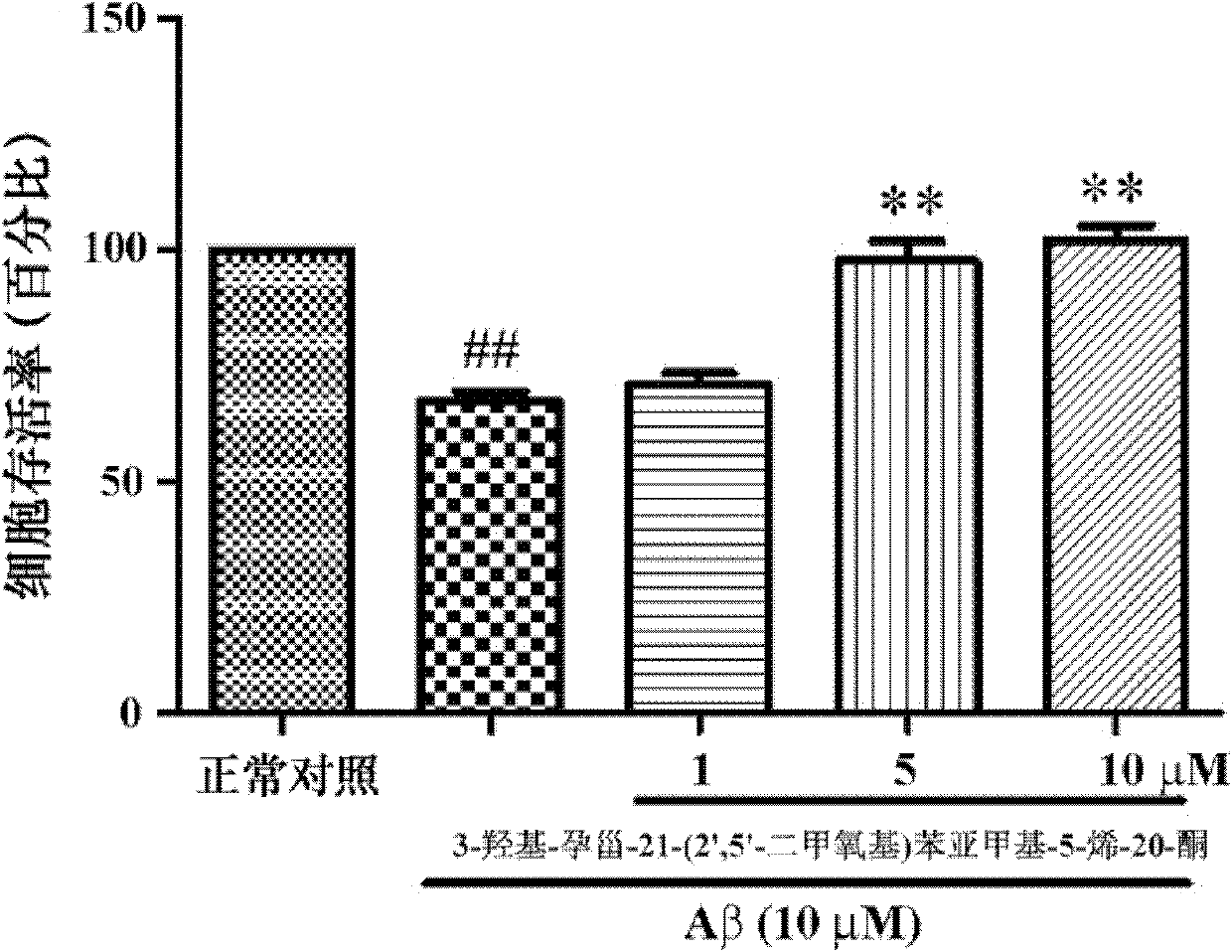
[0033] Example 2: In Vitro Pharmacological Test 1 (Effect of Compounds on Aβ-Induced SH-SY5Y Cell Injury)
[0034] Purpose:
[0035] Observation of the protective effect of 3-hydroxy-pregna-21-(2′,5′-dimethoxy)benzylidene-5-en-20-one on the damage of SH-SY5Y cells induced by Aβ
[0036] experimental method:
[0037] SH-SY5Y was purchased from the National Cell Bank of the United States, using MEM / F12 medium containing 10% fetal bovine serum, 100 U / ml penicillin, and 100 μg / ml streptomycin, at 37°C, 5% CO 2 Routine cultivation in a saturated humidity incubator. Take 2×10 4 The density of cells / well was seeded in a 96-well plate, and the experiment was performed after 24 hours of culture. Aβ treatment: replace with serum-free MEM / F12, add different concentrations of compounds (1-10 μM) shown in structural formula I at the same time, add Aβ with a final concentration of 10 μM after cultivating for 2 hours, continue culturing for 24 hours, then add MTT (final concentration ...
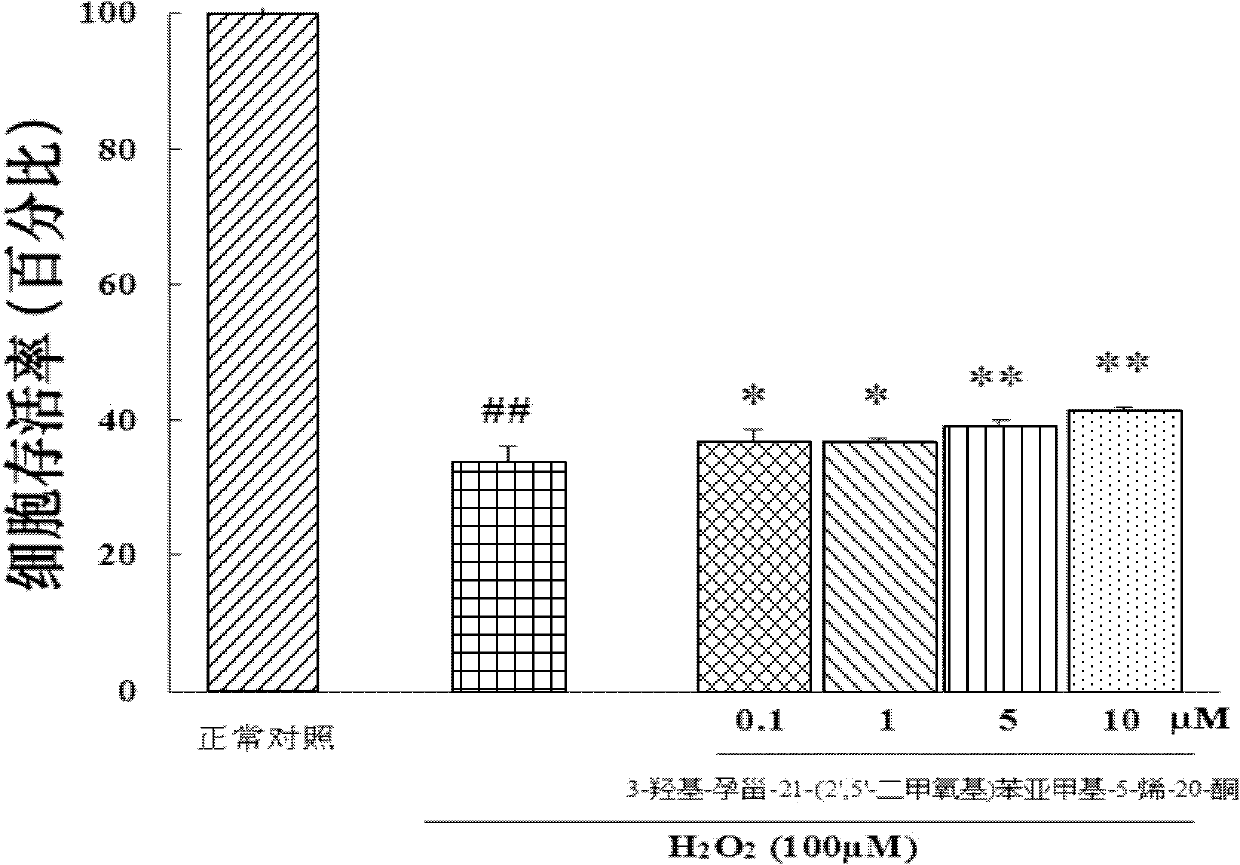
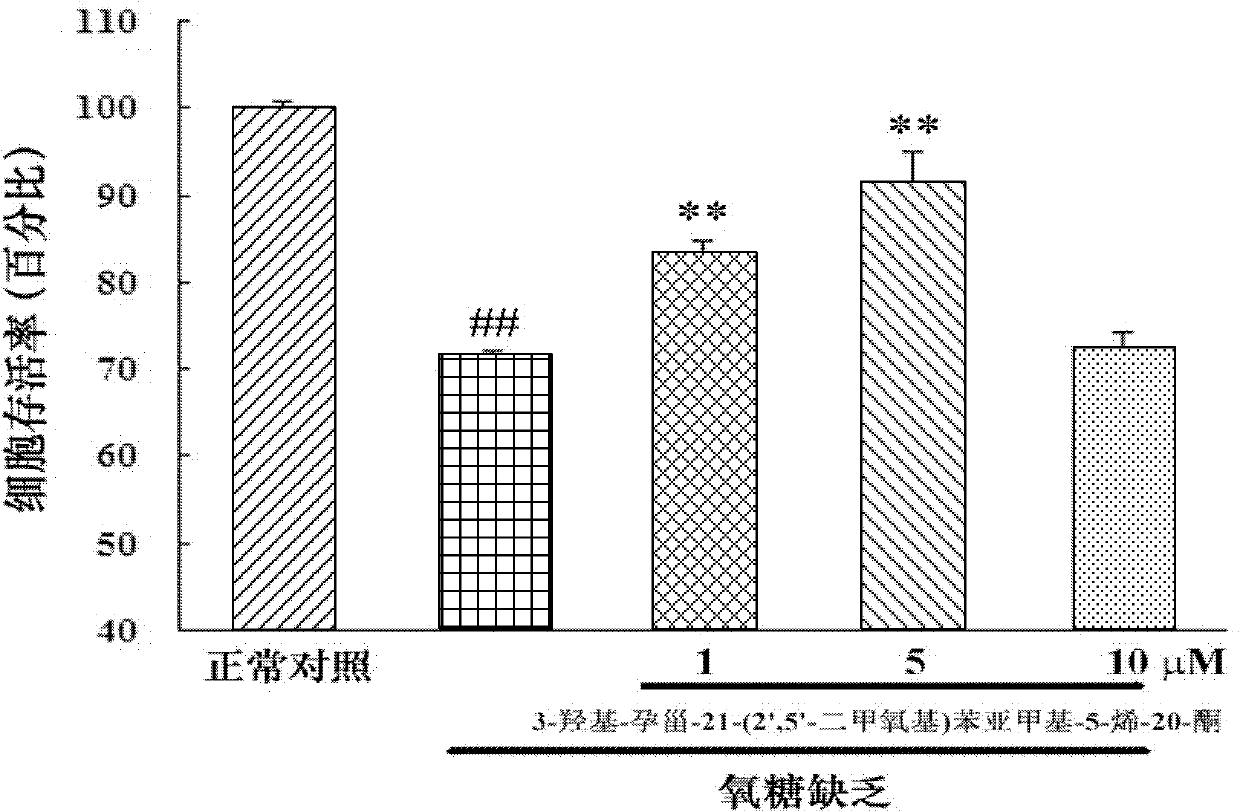
Embodiment 3
[0040] Embodiment 3: in vitro pharmacological test 2 (test compound is to H 2 o 2 inducing cell damage)
[0041] Purpose:
[0042] Observation of the effect of 3-hydroxy-pregna-21-(2′,5′-dimethoxy)benzylidene-5-en-20-one on H 2 o 2 Protective effect of induced SH-SY5Y cell injury
[0043] experimental method:
[0044] Place SH-SY5Y (ATCC) cells in 5% CO 2 cultured in a constant temperature incubator at 37°C. The culture medium was MEM / F12 (1:1), and 10% FBS was added. Change the culture medium every 3-4 days. When the cell fusion rate is about 80%, it is digested with 0.125% trypsin and passaged at a ratio of 1:2. Take the stably growing SH-SY5Y cells, and use 1×10 5 seed / ml density in 96-well plate, 100μl / well, in 5% CO 2 Incubate in a 37°C constant temperature incubator for about 24 hours. Replace with fresh culture medium, and divide the cells into normal control group, H 2 o 2 Injury model group, compound pretreatment group. The compound pretreatment group...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com