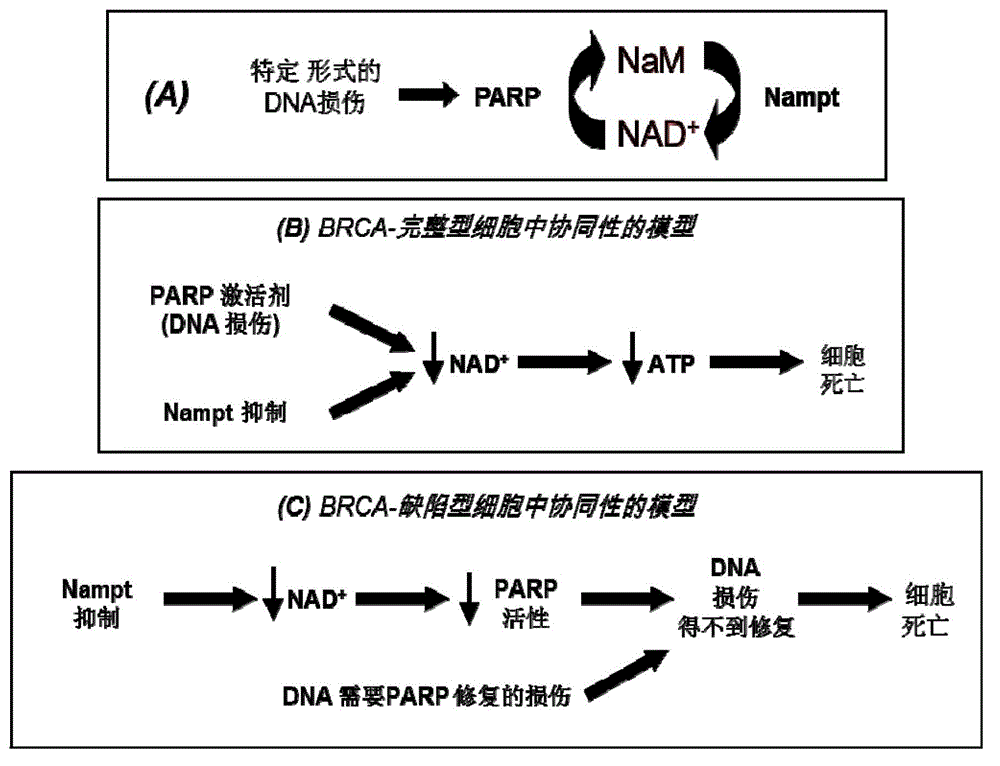
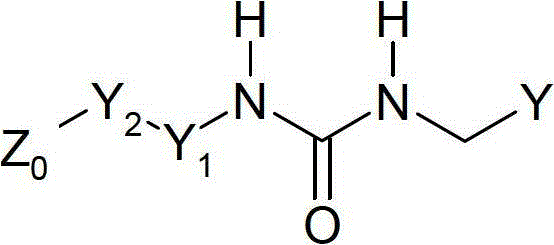
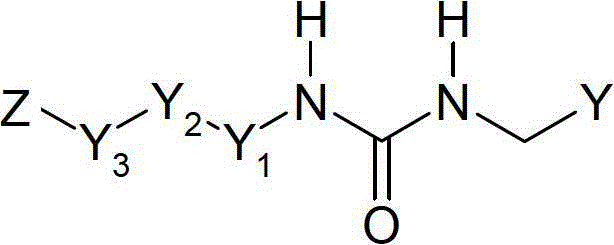
Compounds and therapeutic uses thereof
一种化合物、溶剂合物的技术,应用在药物化学领域,能够解决细胞死亡、消耗等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] 1. Definition
[0029]The term "alkyl" as used herein by itself or as part of another group refers to a saturated aliphatic straight chain or branched chain group having from 1 to 20 carbon atoms (whenever Herein, a numerical range such as "1~20" refers to each integer in the given range; for example, "1~20 carbon atoms" means that the alkyl group can consist of 1, 2 or 3 carbon atoms, or more carbon atoms, up to a total of 20). Alkyl groups may be unsubstituted or have one or more substituents (typically 1 to 3 substituents, except in the case of halogen substituents, eg perchloro). For example, C 1-6 Alkyl refers to an aliphatic hydrocarbon straight chain or branched chain group alkyl containing 1-6 carbon atoms (such as including methyl, ethyl, propyl, isopropyl, butyl, sec-butyl, tert-butyl, 3-pentyl and hexyl, etc.), which may optionally have substituents.
[0030] The term "lower alkyl" as used herein refers to an alkyl group having 1 to 6 carbon atoms.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com