N-4 ( - ( ( 3- ( 2 -amino-4 pyrimidinyl) -2 -pyridinyl) oxy) phenyl) -4- (4-methyl-2-thienyl) -1-phthalazinamine for use in the treatment of antimitotic agent resistant cancer
A pyrimidinyl and pyridyl-based technology, applied in the fields of organic active ingredients, antineoplastic drugs, non-living medical raw materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Step 2: 4-(2-(4-Aminophenoxy)pyridin-3-yl)pyrimidin-2-amine
[0045] To a resealable tube was added 4-aminophenol (1.3 g, 12 mmol), cesium carbonate (7.8 g, 24 mmol) and DMSO (16 ml, 0.75 M). The mixture was heated to 100°C for 5 minutes, then 4-(2-chloropyridin-3-yl)pyrimidin-2-amine (2.5 g, 12 mmol) was added and the reaction mixture was heated to 130°C overnight. After completion (as identified by LCMS), the reaction mixture was cooled to RT and diluted with water. The resulting precipitate was filtered, and the solid was washed with water and diethyl ether. Then in 9:1 CH 2 Cl 2 : Dissolve solids in MeOH with 9:1 CH 2 Cl 2 :MeOH was passed through a pad of silica gel as eluent. The solvent was concentrated in vacuo to afford the desired product 4-(2-(4-aminophenoxy)pyridin-3-yl)pyrimidin-2-amine. MS m / z = 280 [M+1] + . C 15 h 13 N 5 O calculated value: 279.30.
[0046] Step 3: 1-Chloro-4-(4-methylthiophen-2-yl)phthalazine
[0047] 1,4-Dichloroph...
Embodiment 2
[0060] Washing and Fixing Solutions
[0061]
[0062] Reagent
[0063]
[0064] Laboratory equipment, supplies, software
[0065] Desktop refrigerated centrifuge Allegra X-15R centrifuge, Beckman Coulter, Fullerton, CA 92834 GraFit v5 software Erithacus Software, Horley Surrey, RH6 9YJ, UK XLfit 4.2 software Excel, Microsoft Inc., USA GraphPad Prism v5 GraphPad Software, Inc. 2236 Avenida de la Playa, La Jolla, CA 92037 Flow Cytometry Becton Dickinson LSRII flow cytometer, BD Biosciences, San Jose, CA 95131 96-well tissue culture plate, flat bottom low evaporation lid, sterile Cat. No. 3595, lot number not provided, Corning Incorp. Life Sciences, Lowell MA 01851 12-well tissue culture plate, flat bottom low evaporation lid, sterile Cat. No. 353043, Lot No. not provided, BD Labware, Franklin Lakes, NJ 07417 6-well tissue culture plate, flat bottom low evaporation lid, sterile Cat. No. 353046, Lot No. not provided...
Embodiment 3
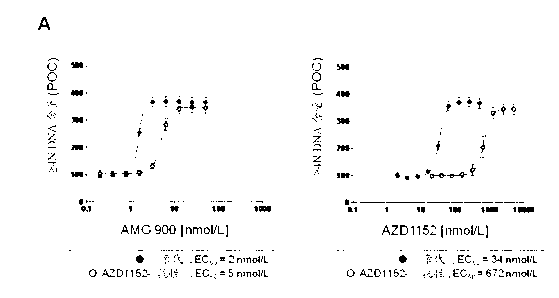
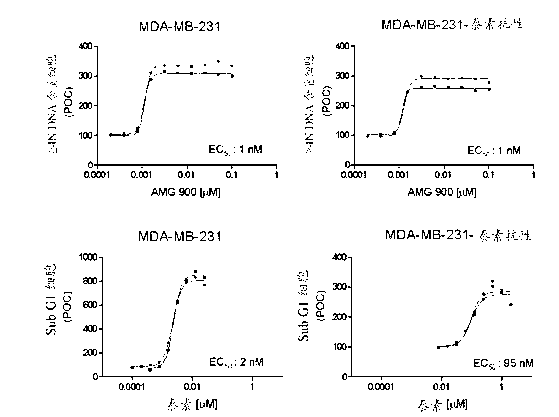
[0113] (ND) Not determined, resistance (r) defined as ≥10-fold loss of potency (EC50 value) compared to taxane-sensitive tumor cell lines
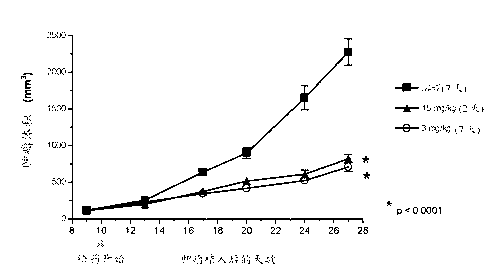
[0114] The effect of AMG 900 was determined on the multidrug resistant cell line MES-SA Dx5 grown in vivo as tumor xenografts. Mice were orally administered AMG 900 at 15 mg / kg twice a day or 3.0 mg / kg twice a day for 2 consecutive days a week during the study period. Dosing was initiated when tumors were established (10 days after tumor implantation). AMG 900 treatment with both AMG 900 doses and regimens resulted in statistically significant tumor growth inhibition compared to vehicle control group (Figure 4; p < 0.0001, Dunnett's post hoc test). Comparable tumor growth inhibition was also surprisingly achieved with similar treatment regimens in 2 other resistance models (HCT15 and NCI-H460-taxol resistance[r]) (see Table 4).
[0115] Figure 4: AMG 900 inhibits the growth of established MES-SA Dx5 xenograft tumors
[0116]
[0117]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com