Application of benzoazepine compounds in preparation of drugs for prevention or treatment of epilepsy
A benzoazepine and compound technology, applied in the field of drugs for preventing or treating epilepsy, can solve problems such as poor selectivity, and achieve the effects of preventing excessive activation, prolonging activation time, and highly specific
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment one: preparation embodiment
[0035] 1 H-NMR was measured with a Varian Mercury AMX300 instrument; MS was measured with a VG ZAB-HS or VG-7070 instrument, and all were EI sources (70ev) unless otherwise noted; all solvents were re-distilled before use, and no The water solvents were all obtained by drying according to standard methods; except for the instructions, all reactions were carried out under nitrogen protection and followed by TLC, and the post-treatment was washed with saturated sodium chloride aqueous solution and dried with anhydrous sodium sulfate; the purification of the product Unless otherwise specified, silica gel (200-300 mesh) column chromatography was used; among them, silica gel (200-300 mesh) was produced by Qingdao Ocean Chemical Factory, and GF254 thin-layer silica gel plate was produced by Yantai Jiangyou Silica Gel Development Co., Ltd.
[0036] 1. Synthesis of Compound S1
[0037] See the reaction below:
[0038]
[0039] Amon...
Embodiment 2
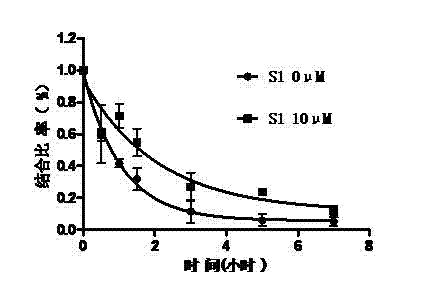
[0131] Example 2 Allosteric regulation of sigma-1 receptors by benzazepine compounds
[0132] experimental method
[0133] (1) animals
[0134] Take healthy adult male SD rats (180-200g), five in one cage, and raise them in an environment with a temperature of 21±2°C, normal day and night (light from 0700h-1900h), and free access to food and water.
[0135] (2) Cell membrane preparation of animal brain tissue
[0136] After the rats were sacrificed by decapitation, the brain tissue was isolated. Then add 10 times the volume of Kreb's solution and homogenize with a glass homogenizer. The homogenate was centrifuged at 4°C for 10 minutes. Aspirate the supernatant, and then centrifuge again for 10 minutes (centrifugal force 1000g, 4°C). Aspirate the supernatant again, centrifuge at a centrifugal force of 35000g for 30 minutes. Finally, the pellet was resuspended in Kreb's solution.
[0137] (3) Receptor binding experiment
[0138] First, the membrane components are diluted...
Embodiment 3
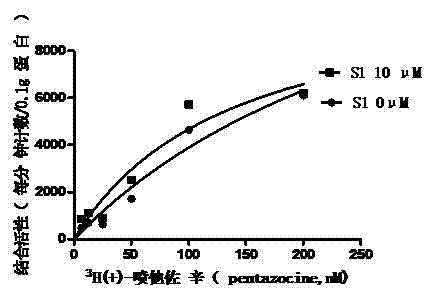
[0146] Example 3 Effect of S1 on the saturation curve of sigma-1 receptor
[0147] experimental method
[0148] In this example only the concentration of the radioligand (5-400nM) was varied. The procedures of experimental animals, cell membrane preparation, and radioactivity measurement are the same as in Example 1. And according to the following formula to fit the saturation curve, calculate its binding constant (Kd):
[0149] B = B max × L K d + L
[0150] where B is the number of receptors bound to the radioligand per unit tissue (mg). Bmax is the maximum number of receptors per unit tissue (mg); Kd is the binding constant; L is 3 H(+)-pentazocine concentration.
[0151] result
[0152] As shown in Figure 1, S1 can shift the saturation curve to th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com