Multifunctional modified dibenzofuran-based phosphineoxy compound and preparation method and application thereof
The technology of furyl bisphosphine and diphenylphosphine oxide is applied in the multifunctional modification of dibenzofuryl bisphosphine oxide compound and the fields of preparation and application thereof, which can solve the problem of high injection/transmission capability starting voltage, and achieves Excellent effect of good carrier injection/transport capability, good thermodynamic stability, driving voltage, and stability of luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0020] Specific embodiment 1: This embodiment provides a multifunctional modified dibenzofuryl bisphosphine oxide compound, which is a derivative of a compound with the chemical name 4,6-bis(diphenylphosphinooxy)dibenzofuran , the structural formula of the multifunctional modified dibenzofuryl bisphosphine oxide is:
[0021]
[0022] Wherein, X described in the structural formula, Y structure is as follows:
[0023] (1) X is Y is hydrogen or (2) X is Y is hydrogen or (3) X is Y is hydrogen or (4) X is Y is hydrogen or
[0024] The structural formula of the 4,6-bis(diphenylphosphinooxy)dibenzofuran compound described in this embodiment is:
[0025]
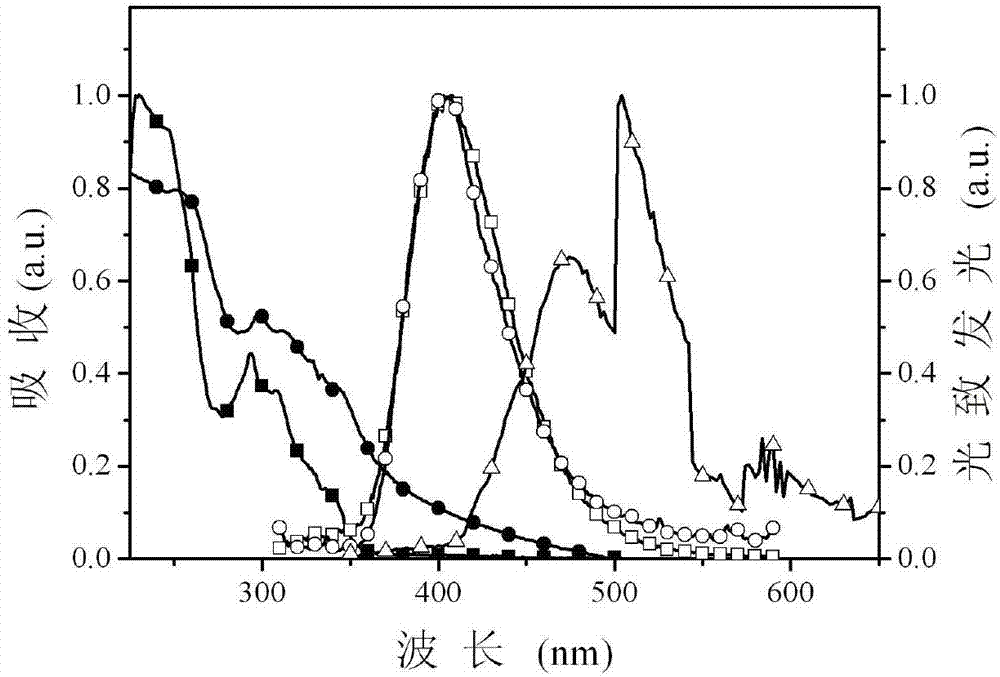
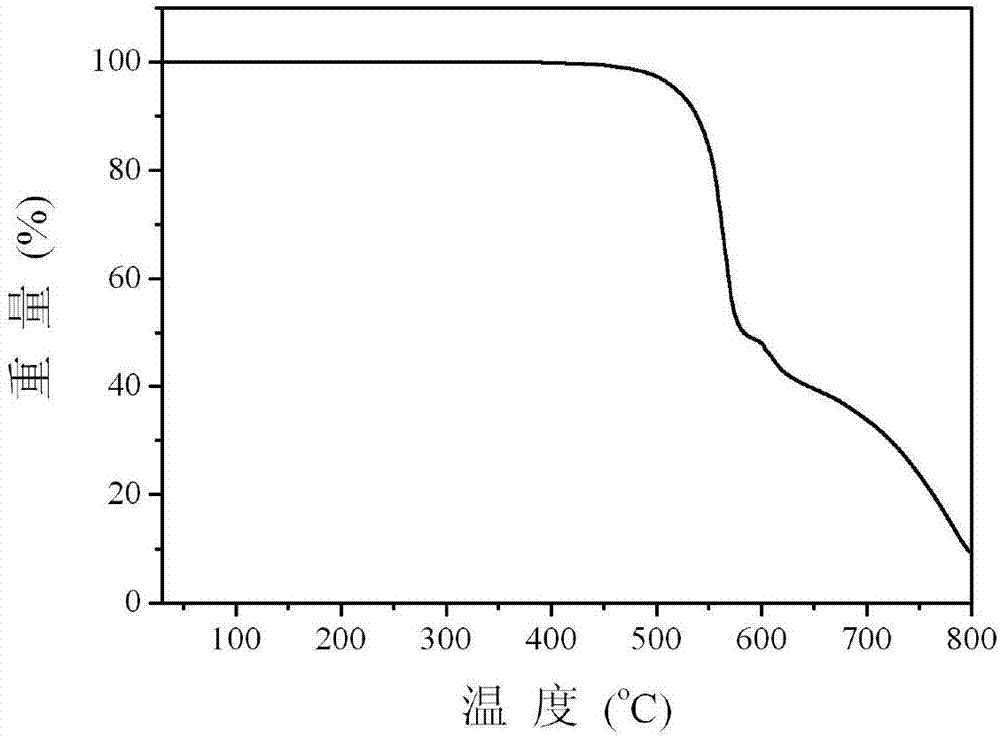
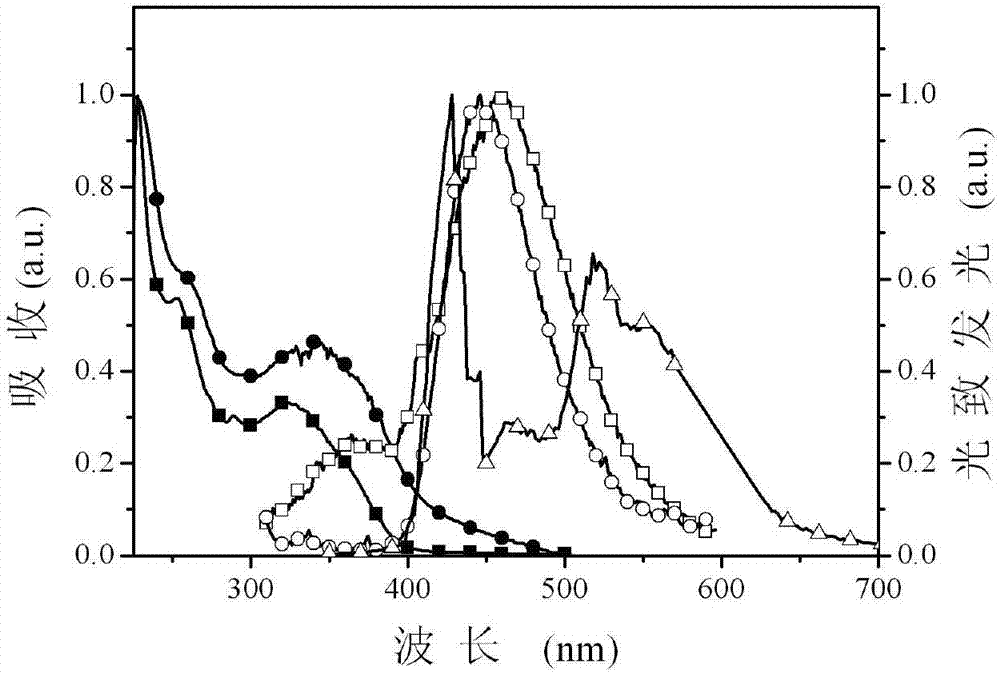
[0026] The multifunctional modified dibenzofuryl bisphosphine oxide compound provided by this embodiment can not only maintain a high triplet energy level, ensure sufficient energy transfer from the host to the guest, but also have good carrier injection / transport capabilities , so as to achieve the purpose ...
specific Embodiment approach 2
[0027] Specific embodiment two: The preparation method of the multifunctional modified dibenzofuryl bisphosphine oxide provided by this embodiment is specifically completed according to the following steps:
[0028] The preparation method of the multifunctional modified dibenzofuryl bisphosphine oxide compound is completed according to the following steps:
[0029] 1. Weigh 4,6-bis(diphenylphosphinooxy)dibenzofuran and dissolve it in concentrated sulfuric acid, add N-bromosuccinimide, stir and react at room temperature for 2h~6h, after the reaction , with CH 2 Cl 2 and H 2 O is extracted, the organic phase is dried with anhydrous sodium sulfate, the organic phase solvent is spin-dried, and purified by column chromatography using ethyl acetate as eluent to obtain 2-bromo-4,6-bis(diphenylphosphine oxy)dibenzofuran or 2,8-dibromo-4,6-bis(diphenylphosphineoxy)dibenzofuran, wherein the volume of concentrated sulfuric acid is equal to that of 4,6-bis(diphenylphosphine The ratio ...
specific Embodiment approach 3
[0042] Specific embodiment three: the difference between this embodiment and specific embodiment two is: N-bromosuccinimide and 4,6-bis(diphenylphosphinoxy)dibenzo The molar ratio of furan is 1:1 or 2:1. Others are the same as in the second embodiment.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cracking temperature | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com