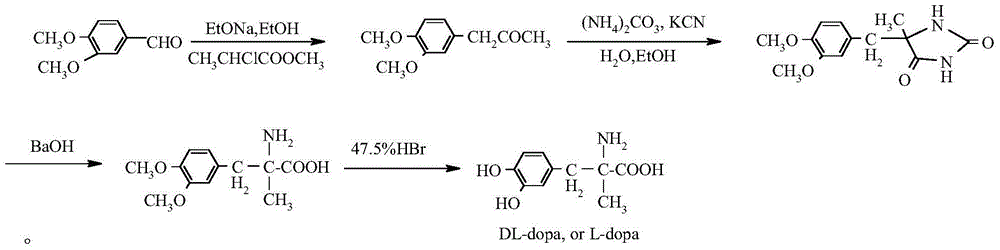
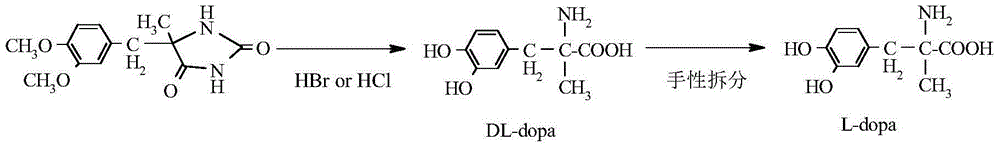
Method for preparing methyldopa by directly hydrolyzing 5-methyl-5-(3,4-dimethoxybenzyl)hydantoin with acid
A technology of dimethoxybenzyl and hydantoin, applied in chemical instruments and methods, cyanide reaction preparation, organic compound preparation, etc., can solve the problems of purity, yield decrease, and increase of by-products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] In a three-necked flask equipped with a thermometer, a stirrer and a reflux condenser, add 4.3g (0.016mol) of 5-methyl-5-(3,4-dimethoxybenzyl)hydantoin, add 36% 24.3 g (0.24 mol) of concentrated hydrochloric acid in an oil bath was then slowly heated to 65-70°C (40 minutes) and stirred for 6 hours, then heated to 100-110°C (to achieve vigorous reflux) and stirred for 24 hours. After the reaction was completed, N was directly introduced into the reaction mixture slowly. 2 , while extracting excess HCl by distillation under reduced pressure. Adjust the pH of the solution with ammonia water and solids appear, stir until the pH is constant. Then, carefully adjust the pH to 6, let stand for 1 hour, and filter the solid. The solid was washed with 4×25ml of acetone to obtain a white solid. It was then vacuum dried at 50°C for 5 hours. Obtain product DL-2-amino-3-(3,4-dihydroxyphenyl)-2-methyl-propionic acid (C 10 h 13 NO 4 ·H 2 o 1.5 ) is 3.57 grams of methyldopa, yie...
Embodiment 2
[0034] In a three-necked flask equipped with a thermometer, a stirrer and a reflux condenser, add 4.3g (0.016mol) of 5-methyl-5-(3,4-dimethoxybenzyl)hydantoin, adding 47% 41.4 g (0.24 mol) of hydrobromic acid, then slowly heated in an oil bath to 65-70°C (40 minutes), stirred and reacted for 6 hours, then heated to 100-120°C (reaching vigorous reflux), stirred and reacted for 18 hours. After the reaction was completed, N was directly introduced into the reaction mixture slowly. 2 , while extracting excess hydrobromic acid by distillation under reduced pressure. Adjust the pH of the solution with ammonia water and solids appear, stir until the pH is constant. Then, carefully adjust the pH to 6, let stand for 1 hour, and filter the solid. The solid was washed with 4×25ml of acetone to obtain a white solid. It was then vacuum dried at 50°C for 5 hours. Obtain product DL-2-amino-3-(3,4-dihydroxyphenyl)-2-methyl-propionic acid (C 10 h 13 NO 4 ·H 2 o 1.5) is 3.63 grams of m...
Embodiment 3
[0036] In a three-necked flask equipped with a thermometer, a stirrer and a reflux condenser, add 4.3g (0.016mol) of 5-methyl-5-(3,4-dimethoxybenzyl)hydantoin, adding 47% 20.7 g (0.12 mol) of hydrobromic acid. Then the oil bath was slowly heated to 65-70°C (40 minutes) and stirred for 6 hours, and then heated to 100-120°C (to achieve vigorous reflux), and stirred for 24 hours. After the reaction was completed, N was directly introduced into the reaction mixture slowly. 2 , while extracting excess hydrobromic acid by distillation under reduced pressure. Adjust the pH of the solution with ammonia water and solids appear, stir until the pH is constant. Then, carefully adjust the pH to 6, let stand for 1 hour, and filter the solid. The solid was washed with 4×25ml of acetone to obtain a white solid. It was then vacuum dried at 50°C for 5 hours. Obtain product DL-2-amino-3-(3,4-dihydroxyphenyl)-2-methyl-propionic acid (C 10 h 13 NO 4 ·H 2 o 1.5 ) namely methyldopa 3.45 gr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com