Method for acquiring regeneration seedlings of dove trees through tissue culture by taking dove tree leaves as explants
A tissue culture and explant technology, applied in plant regeneration, botanical equipment and methods, horticultural methods, etc., can solve problems such as low efficiency and destruction of endangered plants of Dove tree, achieving no seasonal restrictions, good overall efficiency, The effect of a large number of rooting strips
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
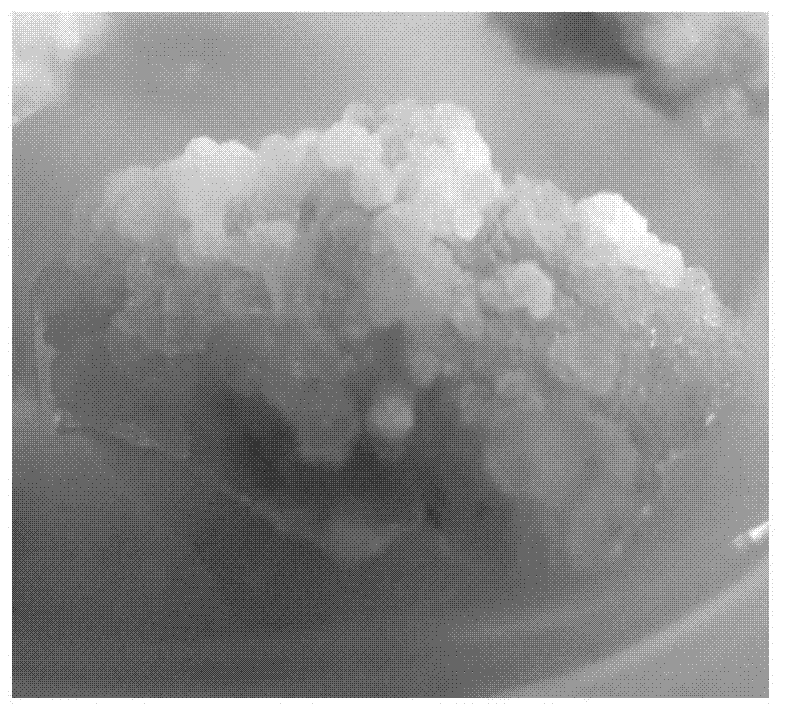
[0037] In this embodiment, young and tender Davidia involucrata leaves are collected from the adult Davidia involucrata plant of Yinchanggou Scenic Area, Pengzhou City, Sichuan Province (see figure 1 ), the sterilization operation of the young Davidia involucrata leaves is as follows: the detergent (White Cat brand) is prepared into a dilution with tap water, and the volume ratio of the detergent to tap water is 1:1000, and the collected young Soak the leaves of Davidia involucrata in the detergent diluent for 10 minutes, take them out and rinse them with tap water for 1 hour to clean the surface debris, then disinfect them with ethanol with a mass concentration of 75% for 30 seconds under aseptic conditions, and then put in 0.1% ethanol Disinfect in mercuric chloride aqueous solution for 4 min, then take it out and rinse it with sterile water for 5 times to complete the sterilization. Cut the sterilized leaves of Davidia involucrata into about 1 cm in size with scissors 2 Sm...
Embodiment 2
[0054] In this embodiment, young leaves of Davidia involucrata are collected from adult Davidia involucrata plants in Mount Emei Scenic Area, Emeishan City, Sichuan Province, and the sterilization operation of the young leaves of Davidia involucrata is the same as that in Example 1. Cut the sterilized leaves of Davidia involucrata into about 1 cm in size with scissors 2 Small pieces are used for inoculation.
[0055] In the present embodiment, the method steps of obtaining the regenerated seedlings of Davidia involucrata through tissue culture using the leaves of Davidia involucrata as explants are as follows:
[0056] (1) Obtained callus from leaves of Davidia involucrata
[0057] Use a 100ml Erlenmeyer flask, fill each bottle with about 40ml of callus induction medium, inoculate 5~6 small pieces of sterilized leaves of Davidia involucrata (the back of the leaves face up), and culture in the dark for 28 days, and the leaves of Davidia involucrata are healed Wounded tissue, ...
Embodiment 3
[0071] In this example, young Davidia involucrata leaves were collected from adult Davidia involucrata plants in Longcanggou Nature Reserve, Yingjing County, Ya'an City, Sichuan Province, and the sterilization operation of the young Davidia involucrata leaves was the same as in Example 1. Cut the sterilized leaves of Davidia involucrata into about 1 cm in size with scissors 2 Small pieces are used for inoculation.
[0072] In the present embodiment, the method steps of obtaining the regenerated seedlings of Davidia involucrata through tissue culture using the leaves of Davidia involucrata as explants are as follows:
[0073] (1) Obtained callus from leaves of Davidia involucrata
[0074] Use a 100ml Erlenmeyer flask, fill each bottle with about 40ml of callus induction medium, inoculate 5~6 small pieces of sterilized leaves of Davidia involucrata (the back of the leaves face up), and culture in the dark for 30 days, and the leaves of Davidia involucrata are healed injured ti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com