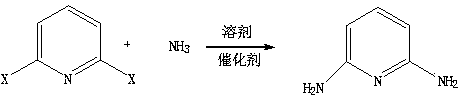
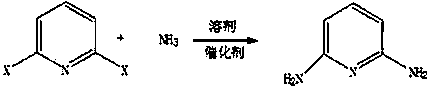
Method for synthesizing 2,6-diamino pyridine
A diaminopyridine and synthesis method technology, applied in the field of 2,6-diaminopyridine synthesis, can solve the problems of low synthesis process yield and many by-products, and achieve good product selectivity, easy operation, and simple process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] In the autoclave, add 1mol of 2,6-dichloropyridine, the activated loading capacity is 10% nano-ruthenium carbon catalyst and 800 ml of quinoline, close the autoclave, feed 20mol of liquid ammonia, close After the reactor, the temperature was raised to 350 ° C, and the reaction was stirred for 12 hours. After the reaction, the excess liquid ammonia was drained, 300 ml of water was added, stirred and cooled to 0-5 ° C, 89 g of solids were obtained by filtration, and 99.1% of The 2,6-diaminopyridine product was 81.8 grams, with a melting point of 121.1-122.3°C and a yield of 75%.
Embodiment 2
[0017] Add 1mol of 2,6-bromochloropyridine in the autoclave, the nanometer ruthenium carbon catalyst and the quinoline of 800 milliliters through the activated loading capacity of 10%, close the high pressure reactor, pass into 20mol of liquid ammonia, close After the reactor, the temperature was raised to 350°C and the reaction was stirred for 12 hours. After the reaction, excess liquid ammonia was drained, 300 milliliters of water was added, stirred and cooled to 0-5°C, filtered to obtain 95 grams of solid, and purified with toluene to obtain 99.3% of 2 , 83.5 grams of 6-diaminopyridine product, melting point 121.3-122.5°C, yield 76.6%.
Embodiment 3
[0019] Add 1mol of 2,6-bromochloropyridine in the autoclave, the nanometer ruthenium carbon catalyst and the quinoline of 800 milliliters through the activated loading capacity of 10%, close the autoclave reactor, pass into the liquid ammonia of 20mol, close After the reactor, the temperature was raised to 350 ° C, and the reaction was stirred for 12 hours. After the reaction, the excess liquid ammonia was drained, 300 ml of water was added, stirred and cooled to 0-5 ° C, 102 g of solids were obtained by filtration, and 99.3% of 92.8 g of 2,6-diaminopyridine product, melting point 122.3-122.7°C, yield 85.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com