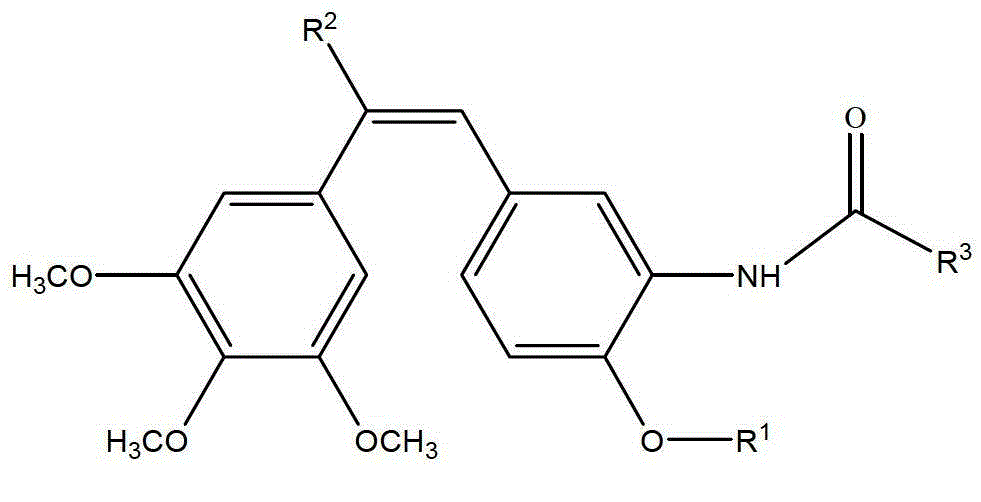
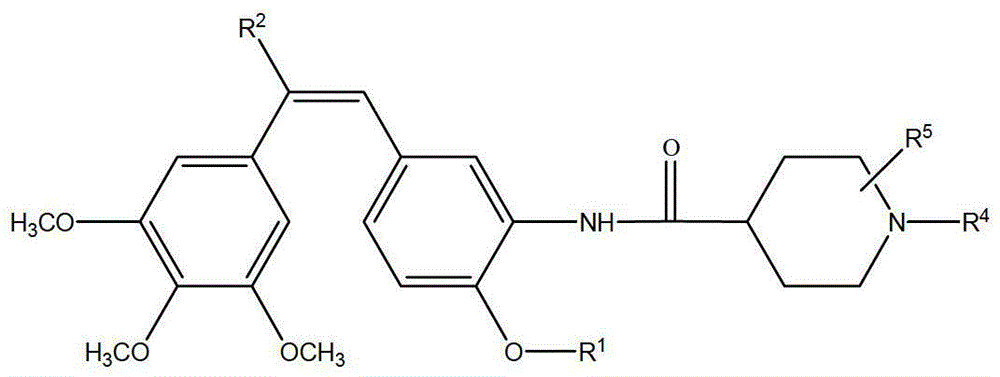
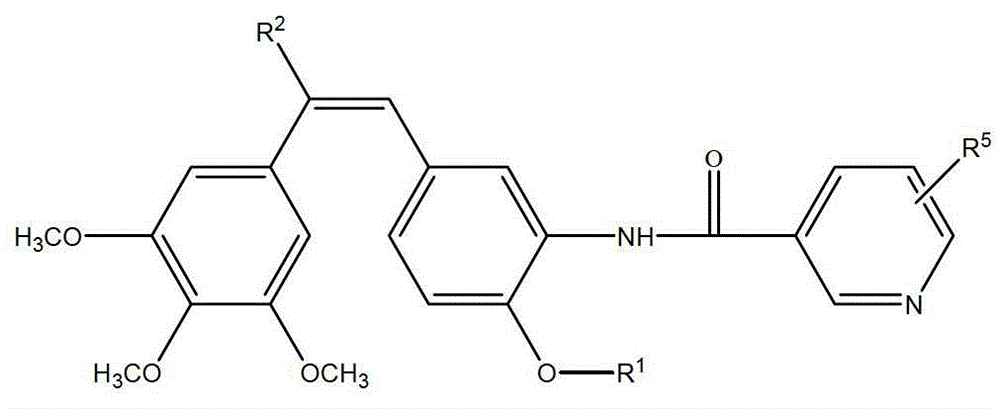
Synthesis of amino combretastatin derivative and application of amino combretastatin derivative as oral antitumour drug
An amino and drug technology, applied in the field of medicine, can solve problems such as inconvenient use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] (Z)-1-(3,4,5-trimethoxyphenyl)-2-(3-(N-methylpiperidine-4-formyl)amino-4-ethoxyphenyl)ethylene synthesis
[0070]
[0071] Step 1: Synthesis of Trimethoxybenzyltriphenylphosphine Bromide
[0072] Dissolve 4Kg of 3,4,5-trimethoxybenzaldehyde in absolute ethanol, stir, and add 1.4Kg of sodium borohydride in small amounts at a temperature below 35°C. After the addition was complete, the reaction was carried out overnight. After the reaction was complete, ethanol was removed by rotary evaporation. Ethyl acetate (10L×4) was added for extraction, the ethyl acetate layers were combined, dried over anhydrous magnesium sulfate, filtered, and the ethyl acetate was distilled off under reduced pressure to obtain 3200ml of the product.
[0073] Dissolve 3200ml of 3,4,5-trimethoxybenzyl alcohol in 20L of toluene, stir, cool down to -5-0°C, add 1L of phosphorus tribromide dropwise, and control the temperature at -5-0°C. After the dropwise addition was completed, react at low te...
Embodiment 2
[0083] (Z)-1-(3,4,5-trimethoxyphenyl)-2-(3-(N-methylpiperidine-4-formyl)amino-4-ethoxyphenyl)ethylene lemon Salt preparation
[0084] (Z)-1-(3,4,5-trimethoxyphenyl)-2-(3-(N-methylpiperidine-4-formyl)amino-4-ethoxyphenyl)ethylene Add 20g into 200ml of acetone, heat to dissolve, add 10g of citric acid, continue to heat and reflux for 3h, filter the precipitate after cooling, and recrystallize with ethanol to obtain the compound shown in the title.
[0085] Utilize different raw materials, by the similar method of embodiment 1, synthesized following various compounds respectively:
[0086]
[0087]
[0088]
[0089]
[0090]
[0091]
Embodiment 31
[0092] Example 31 Pharmaceutical preparation formulation
[0093] The invention provides formulations of pharmaceutical compositions for several diseases related to abnormal proliferation of new blood vessels, and the pharmaceutical compositions mainly include oral preparations such as tablets and capsules. Hereinafter, the compounds shown in the present invention are represented by "active compound".
[0094]
[0095]
[0096]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com