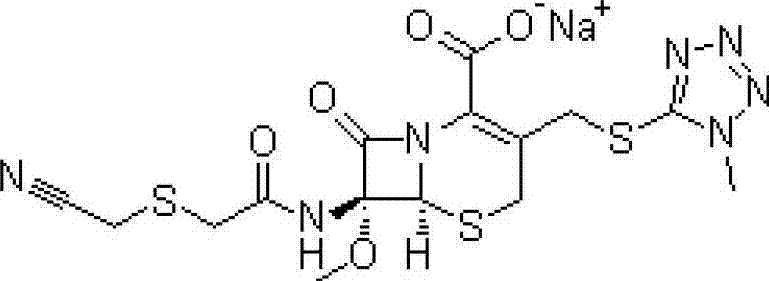
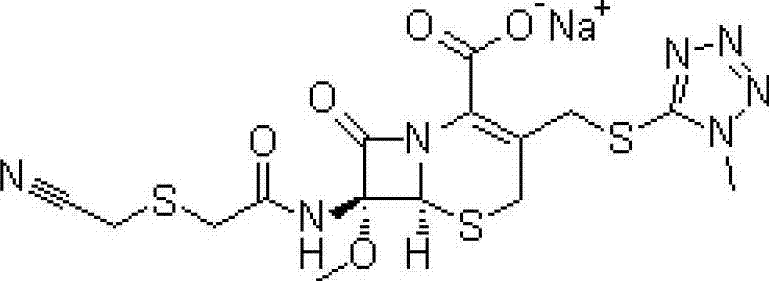
Novel cefmetazole compound and medicine composition thereof
A technology of cefmetazole sodium and cefmetazole, applied in the field of second-generation cephalosporin compounds and pharmaceutical compositions thereof, can solve the problems of increasing the chance of adverse reactions, reducing the content of cefmetazole sodium, increasing total impurities and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A pharmaceutical composition of cefmetazole sodium, the pharmaceutical composition is an injection, and the preparation method of the injection consists of the following steps:
[0026] (1) Dissolve 6g of cefmetazole sodium, 0.05g of mannitol, 0.2g of citrulline and 0.1g of sodium citrate in water for injection, add water to 1000ml, and stir well;
[0027] (2) Add activated carbon to the solution that has been stirred evenly in step (1), stir, adjust pH=6 with sodium hydroxide, decarburize and filter, filter through membrane, and fill;
[0028] (3) Rapidly cool down the solution filled in step (2) to freezing in a freeze dryer, maintain freezing at -40°C to -30°C for 3-5 hours, vacuumize, freeze and vacuum dry for 24 hours, vacuum press Plastic cap, rolled cap, obtains cefmetazole sodium injection.
Embodiment 2
[0030] A pharmaceutical composition of cefmetazole sodium, the pharmaceutical composition is an injection, and the preparation method of the injection consists of the following steps:
[0031] (1) Dissolve 8g of cefmetazole sodium, 0.2g of mannitol, 0.7g of citrulline and 0.5g of sodium citrate in water for injection, add water to 1000ml, and stir well;
[0032] (2) Add activated carbon to the solution that has been stirred evenly in step (1), stir, adjust pH=6 with sodium hydroxide, decarburize and filter, filter through membrane, and fill;
[0033] (3) Rapidly cool down the solution filled in step (2) to freezing in a freeze dryer, maintain freezing at -40°C to -30°C for 3-5 hours, vacuumize, freeze and vacuum dry for 24 hours, vacuum press Plastic cap, rolled cap, obtains cefmetazole sodium injection.
Embodiment 3
[0035] A pharmaceutical composition of cefmetazole sodium, the pharmaceutical composition is an injection, and the preparation method of the injection consists of the following steps:
[0036] (1) Dissolve 9g of cefmetazole sodium, 0.08g of mannitol, 0.5g of citrulline and 0.25g of sodium citrate in water for injection, add water to 1000ml, and stir well;
[0037] (2) Add activated carbon to the solution that has been stirred evenly in step (1), stir, adjust pH=6 with sodium hydroxide, decarburize and filter, filter through membrane, and fill;
[0038] (3) Rapidly cool down the solution filled in step (2) to freezing in a freeze dryer, maintain freezing at -40°C to -30°C for 3-5 hours, vacuumize, freeze and vacuum dry for 24 hours, vacuum press Plastic cap, rolled cap, obtains cefmetazole sodium injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com