Method for preparing sodium stannate from oxide slag and anode slime produced during the production of stannous sulfate
A technology of stannous sulfate and oxide slag, applied in chemical instruments and methods, tin compounds, inorganic chemistry and other directions, can solve the problems of low content of refined tin impurities and high production costs, save energy, improve recovery rate and reduce production costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
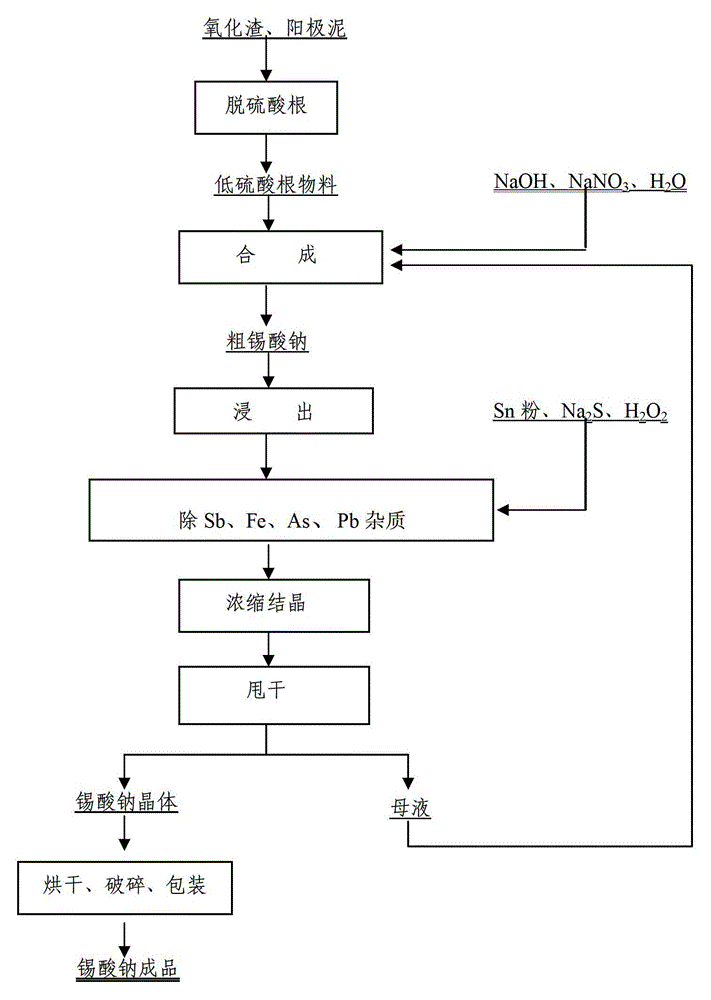
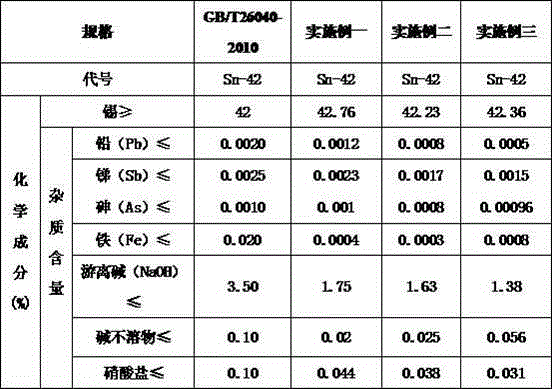
[0032] First, the stannous sulfate oxide slag and anode slime are placed in W Na2CO3 :W SO4 2- =1.10, solid-to-liquid ratio=1:3, reaction time is 60 min, and reaction temperature is 60°C to remove sulfate radicals to obtain oxide slag and anode slime low sulfate radical materials with sulfate radical content of 0.098%. Then these low sulfate content oxide slag, anode slime and NaOH, NaNO 3 、H 2 O is mixed according to the ratio of 1: 1: 2: 0.45: 5, and the oxidation reaction is carried out at 300°C for 45 minutes to generate sodium stannate and ammonia gas. React for 60 min to convert metal tin into sodium stannate as much as possible, and let excess NaNO 3 Decomposition gives crude sodium stannate. Crude sodium stannate was dissolved in hot water at a liquid-solid ratio of 5:1 for leaching to obtain a crude sodium stannate leaching solution with high impurities, and then the solution temperature was 60°C, the reaction time was 60min, and the stirring speed was 20r / min. ...
Embodiment 2
[0034] First, the stannous sulfate oxide slag and anode slime are placed in W Na2CO3 :W SO4 2- =1.18, solid-to-liquid ratio=1:3, reaction time is 90 min, and reaction temperature is 72°C for sulfate removal, to obtain oxide slag and anode slime low-sulfate materials with sulfate content of 0.093%. Then these low sulfate content oxide slag, anode slime and NaOH, NaNO 3 、H 2 O is mixed according to the ratio of 1: 1: 2.20: 0.55: 5, and the oxidation reaction is carried out at 350 ° C for 75 minutes to generate sodium stannate and ammonia gas. React for 100 min to convert metal tin into sodium stannate as much as possible, and let excess NaNO 3 Decomposition gives crude sodium stannate. Crude sodium stannate is dissolved in hot water at a liquid-solid ratio of 5:1 for leaching to obtain a crude sodium stannate leaching solution with high impurities, and then the solution temperature is 75°C, the stirring speed is 25r / min, and the reaction time is 75min. Under the conditions...
Embodiment 3
[0036] First, the stannous sulfate oxide slag and anode slime are placed in W Na2CO3 :W SO4 2- =1.2, solid-to-liquid ratio=1:3, reaction time 120min, reaction temperature 100°C for sulfate removal, to obtain oxidized slag and anode slime low-sulfate materials with sulfate content of 0.089%. Then these low sulfate content oxide slag, anode slime and NaOH, NaNO 3 、H 2 O is mixed according to the ratio of 1: 1: 2.25: 0.6: 5, and the oxidation reaction is carried out at 400°C for 90 minutes to generate sodium stannate and ammonia gas. After the water in the reactant is evaporated to dryness, it is calcined at 1000°C. In-depth reaction for 120 min to convert metal tin into sodium stannate as much as possible, and let excess NaNO 3 Decomposition gives crude sodium stannate. Crude sodium stannate is dissolved in hot water at a liquid-solid ratio of 5:1 for leaching to obtain a crude sodium stannate leaching solution with high impurities, and then the solution temperature is 80°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com