Application of solvent photochromic spiro compound
A spiro compound, chromogenic technology, applied in application, color-changing fluorescent materials, analysis by chemical reaction of materials, etc., can solve problems such as environmental pollution and easy destruction of molecular structure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
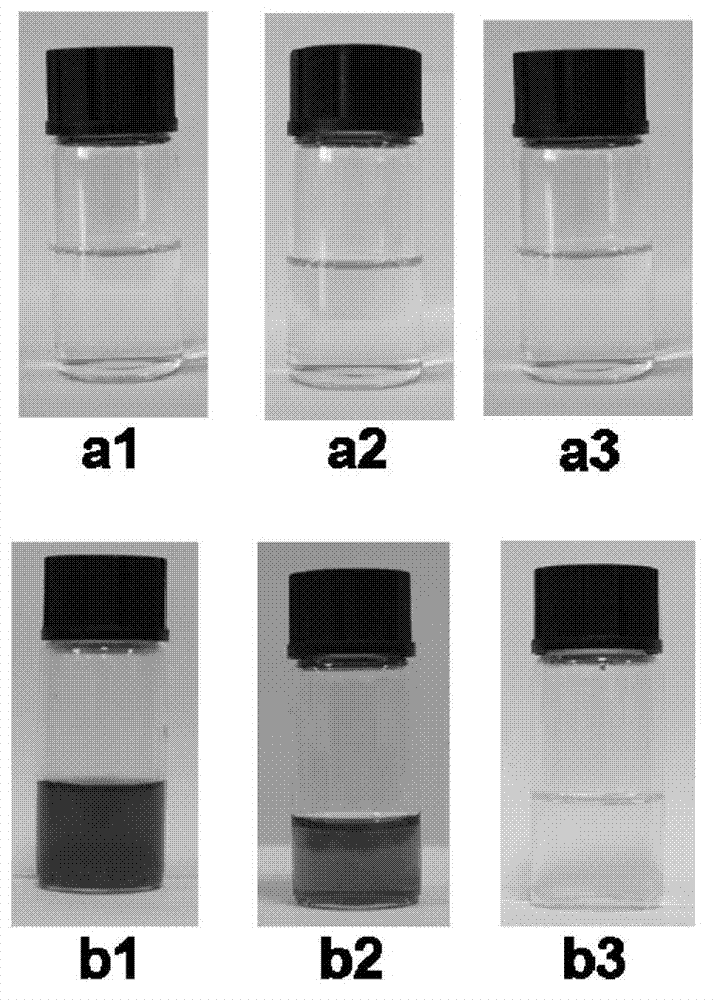
[0033] A1-1 (eg Figure 4 ) is Ar in structural formula 1 1 is phenyl, R 1 , R 2 is methyl, R 3 N, N-dimethylamino, n is an integer 1, and when X is an O atom, a solvochromic spiro compound is obtained, which is colorless in tetrahydrofuran solution ( Figure 7 (a1)), when the ratio of tetrahydrofuran and water is 1:4, it is rose red ( Figure 7 (b1)), this solvent-dependent property can be applied in solvent property indicators, solvent component detection, etc.
Embodiment 2
[0035] A1-2 (eg Figure 4 ) is Ar in structural formula 1 1 is 2-nitrophenyl, R 1 , R 2 is methyl, R 3 N, N-dimethylamino, n is an integer 1, and when X is an O atom, a solvochromic spiro compound is obtained, which is colorless in absolute ethanol solution ( Figure 7 (a2)), blue when the ratio of absolute ethanol and water is 1:1 ( Figure 7 (b2)), this solvent-dependent property can be applied in solvent property indicators, solvent component detection, etc.
Embodiment 3
[0037] A1-3 (eg Figure 4 ) is Ar in structural formula 1 1 is phenyl, R 1 , R 2 is methyl, R 3 is a methyl group, n takes an integer 1, and when X is an O atom, a solvochromic spirocyclic compound is obtained, which is colorless in absolute ethanol solution ( Figure 7 (a3)), yellow when the ratio of absolute ethanol and water is 1:4 ( Figure 7 (b3)), this solvent-dependent property can be applied in solvent property indicators, solvent component detection, etc.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com