Sheet preparation for tissue adhesion
A preparation and freeze-dried tablet technology, applied in the field of tablet preparation and its preparation, can solve the problems of low tensile strength, insufficient tissue adhesion, poor tissue sealing ability, etc., achieve excellent flexibility and elasticity, and improve physical strength and stability effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Embodiment 1: the method for preparing tablet preparation
[0078] For sheet-shaped carriers, bioabsorbable sheets are used. Specifically, for the bioabsorbable sheet, Neoveil 015G (registered trademark) (Gunze; composition: polyglycolic acid; thickness 0.15 mm), Neoveil 03G (registered trademark) (Gunze; composition: polyglycolic acid; thickness 0.3 mm) were used and Neoveil 05G (registered trademark) (Gunze; composition: polyglycolic acid; thickness 0.5 mm).
[0079]Prepare fibrinogen solution I to contain about 12.0% (w / v) fibrinogen, about 90 IU / mL coagulation factor XIII, 1.5% (w / v) human serum albumin, 2.6 %(w / v) Sodium Chloride, 1.8%(w / v) Trisodium Citrate, 0.5%(w / v) Isoleucine, 0.7%(w / v) Glycine, 1.6%(w / v) Arginine Hydrochloride, 1.2% (w / v) Monosodium Glutamate, 0.03% (w / v) Polysorbate 80, 1.0% Glycerin. Fibrinogen solution II is a solution obtained by doubly diluting fibrinogen solution I with water for injection to obtain a solution of about 6.0% (w / v) fi...
Embodiment 2
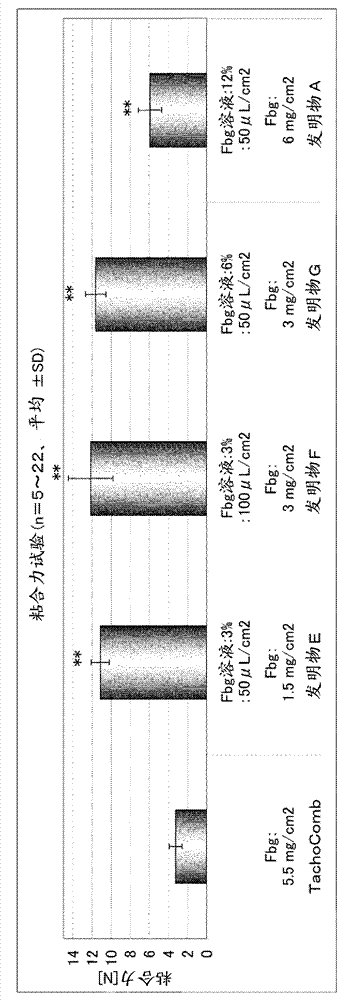
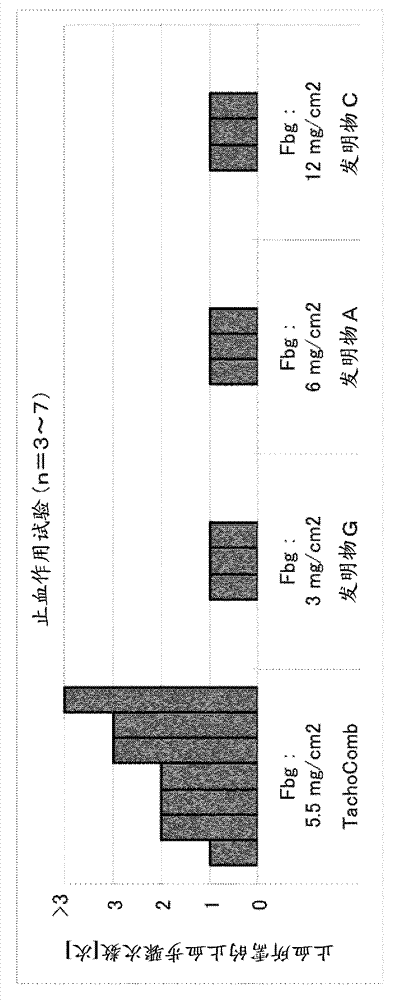
[0092] Embodiment 2: Evaluation test of tablet preparation adhesive force
[0093] For saline, saline of the Japanese Pharmacopoeia (Otsuka Pharmaceutical Co., Ltd., ). For water for injection, water for injection of the Japanese Pharmacopoeia (The Chemo-Sero-Therapeutic Research Institute, ). As a control of the tablet preparation, TachoComb (registered trademark) (CSL Behring) was used.
[0094] Fix two pieces of porcine dermis (adhesive area: 2.5×2.5cm) with a fixing device, and put 50μL / cm 2 Saline was applied to these tablets, and the tablet formulation was placed on top. Immediately after pressing for 5 minutes, one of the fixtures was pulled horizontally, the maximum tensile strength (N: Newton) was measured, and the obtained value was recorded as the adhesive force of the tablet preparation. For TachoComb, the N value is 22, for Invention E / G, the N value is 5, for the Invention A / B / D / F / J / K / L, the N value is 6, for the Invention D, the N value is 8. The resu...
Embodiment 3
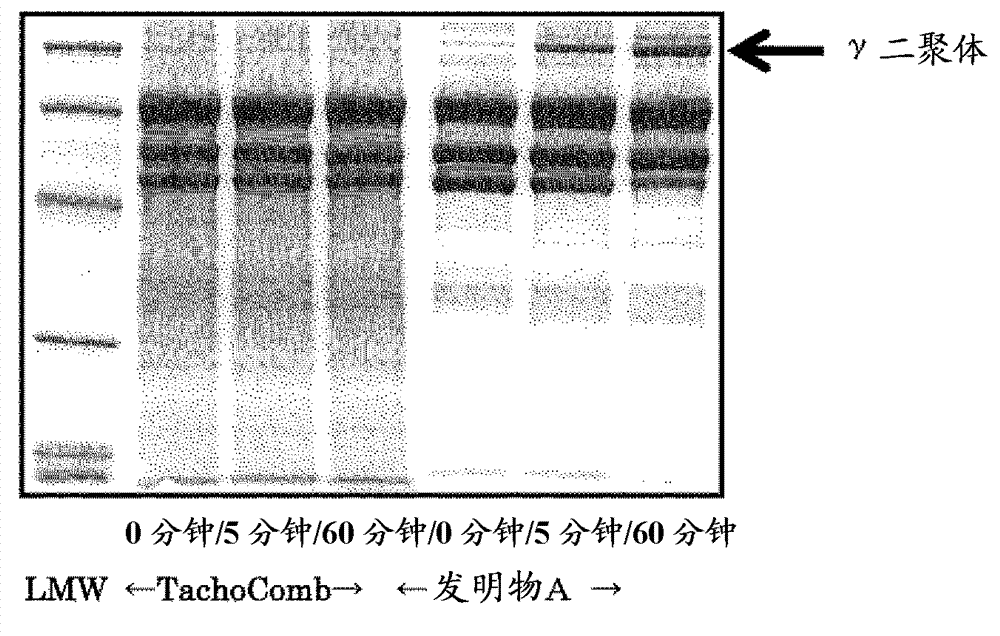
[0097] Embodiment 3: SDS-PAGE of tablet preparation
[0098] [Sample preparation before gelling: 0 minutes]
[0099] TachoComb and Invention B prepared in Example 1 were tested. Prepare 0.5×0.8cm=0.4cm 2 slices and placed in sample tubes. Add the reducing solution to the sample tube. The tablets were allowed to dissolve at room temperature for 48 hours while stirring the solution containing the tablets appropriately. After boiling, electrophoresis, staining (CBB) and destaining were performed.
[0100] [Sample preparation after gelling: 5, 60 minutes]
[0101] TachoComb and Invention A prepared in Example 1 were tested. Prepare 0.5×0.8cm=0.4cm 2 slices and placed in sample tubes. Saline (40 μL) was added to the sample tube to dissolve and undergo gelation. 5 minutes or 60 minutes after the addition of saline and gelation, the reducing solution was added to end the gelation. While stirring the solution containing the gel flakes appropriately, the flakes were allowed...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com