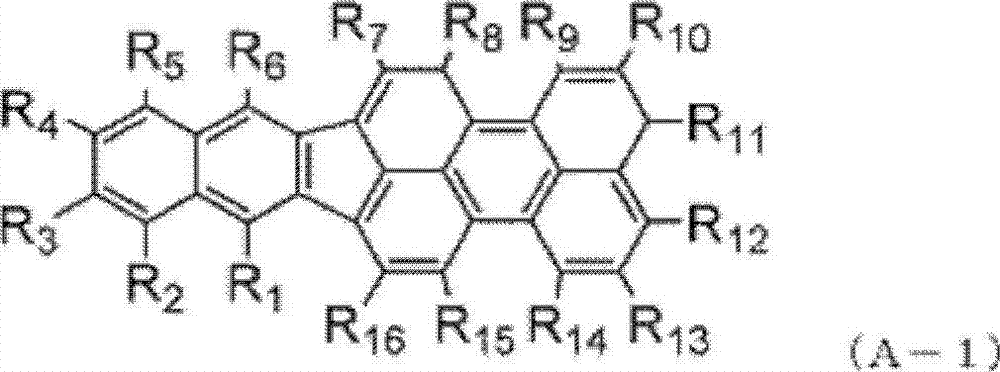
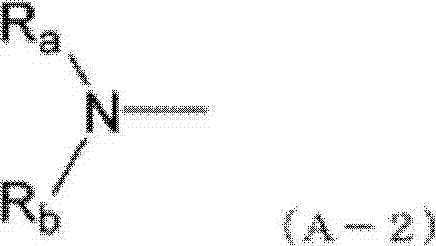
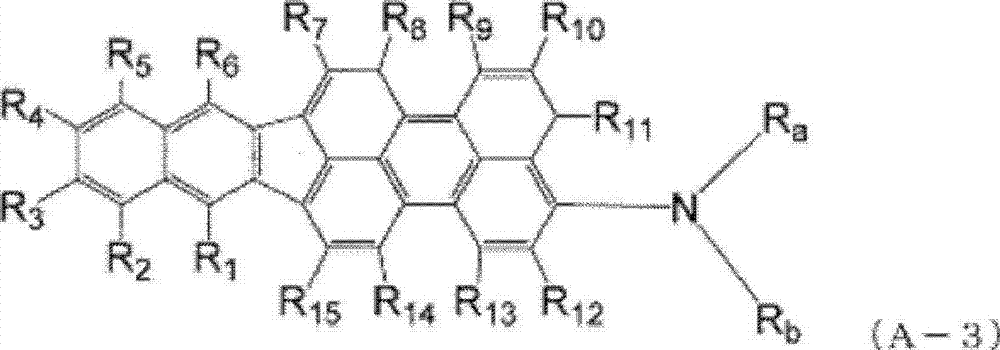
Indenoperylene compound, material for organic thin-film photovotaic cell containing indenoperylene derivative, and organic thin-film photovotaic cell using same
A technology for solar cells and organic thin films, applied in the preparation of perylene derivatives, organic compounds, organic chemistry, etc., can solve the problems of undisclosed perylene derivatives, etc., and achieve the effect of excellent photoelectric conversion characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0224] Embodiment 1 (synthesis of compound A)
[0225] The raw material A of compound A was synthesized in the following procedure.
[0226] [chem 22]
[0227]
[0228] Compound A was synthesized as follows.
[0229] [chem 23]
[0230]
[0231] Under nitrogen atmosphere, make raw material A (1.2g, 8.9mmol), diphenylamine (0.4g, 2.4mmol, 1.2eq.), tris (dibenzylideneacetone) dipalladium (0) (0.03g, 0.03 mmol, 3% Pd), sodium tert-butoxide (0.3g, 2.8mmol, 1.4eq.) was suspended in anhydrous toluene (50ml), and tri-tert-butylphosphine / toluene solution (2.8M, 0.017ml, 0.048mmol , 0.8eq. relative to Pd), reflux was carried out for 7 hours. The reaction mixture was filtered through a pad of silica gel, washing with toluene (100ml). The red oily solid obtained by distilling off the filtrate from the solvent was purified by column chromatography (silica gel / hexane+17% dichloromethane, followed by hexane+33% dichloromethane) to obtain a red solid (1.2 g, 86%).
[0232] Variou...
Embodiment 2
[0236] Embodiment 2 (manufacture of organic thin film solar cell)
[0237] A 25 mm x 75 mm x 0.7 mm thick glass substrate with an ITO transparent electrode was cleaned ultrasonically for 5 minutes in isopropyl alcohol, and then washed with UV ozone for 30 minutes. Load the cleaned glass substrate with transparent electrode wires on the substrate holder of the vacuum evaporation device, on the surface of the side where the transparent electrode wires as the lower electrodes are first formed, pass through the resistor in such a way as to cover the transparent electrodes heated and evaporated with Compound A (p-type material) was formed into a film with a film thickness of 30 nm.
[0238] Then, on the compound A film, evaporated by resistance heating and Make the film thickness 60nm C 60 (n-type material) film formation. Further, as a buffer layer, to A 10 nm bathocuproine (BCP) film was formed. Finally, as a counter electrode, metal Al was vapor-deposited with a film th...
Embodiment 3
[0264] [Synthesis of Compound B]
[0265] Compound B was synthesized according to the following synthetic route.
[0266] [chem 28]
[0267]
[0268] [chem 29]
[0269]
[0270] [Synthesis of raw material B]
[0271]Under a nitrogen atmosphere, 2,2,6,6-tetramethylpiperidine (6.6 g, 47 mmol, 1.5 eq.) was dissolved in anhydrous THF (60 ml) and cooled to -43°C in a dry ice / methanol bath. A n-butyllithium / hexane solution (1.6mol / l, 28ml, 47mmol, 1eq.toTMP) was added dropwise thereto, and stirred at -23°C for 20 minutes. The reaction mixture was cooled to -74°C, triisopropyl borate (15ml, 65mmol, 2eq.) was added, and after 5 minutes, 4-bromotriphenylamine / anhydrous THF solution (8g, 39mmol / 15ml). The reaction mixture was stirred at -74°C to room temperature for 10 hours and left overnight. Cool the reaction mixture in a water bath, slowly add 5% hydrochloric acid aqueous solution (100ml) to inactivate it, dilute it with ethyl acetate (150ml), take out the organic layer...
PUM
| Property | Measurement | Unit |
|---|---|---|
| area | aaaaa | aaaaa |
| absorption wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap