Tricyclic lactones for treatment of cancer
A carbocyclic and heterocyclic technology, applied in the field of new tricyclic compounds, can solve the problems of low activity and no activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
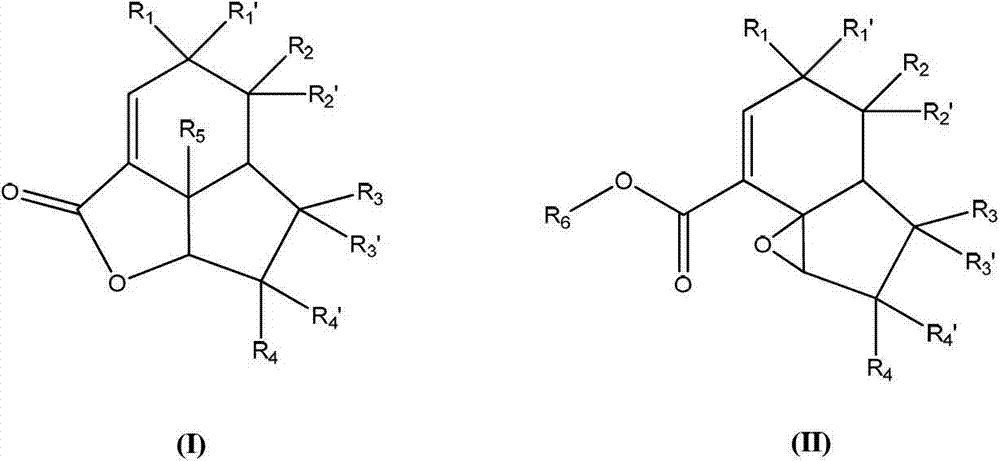
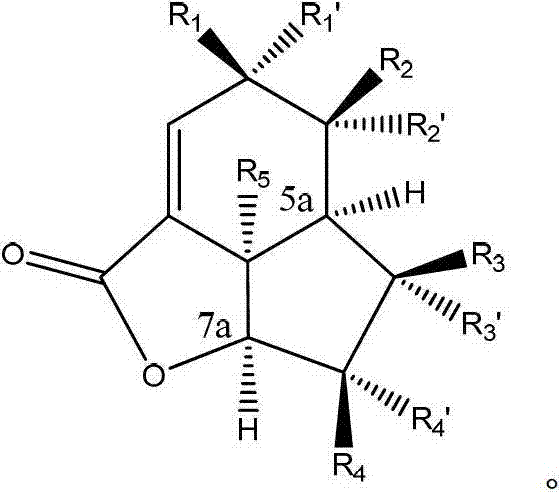
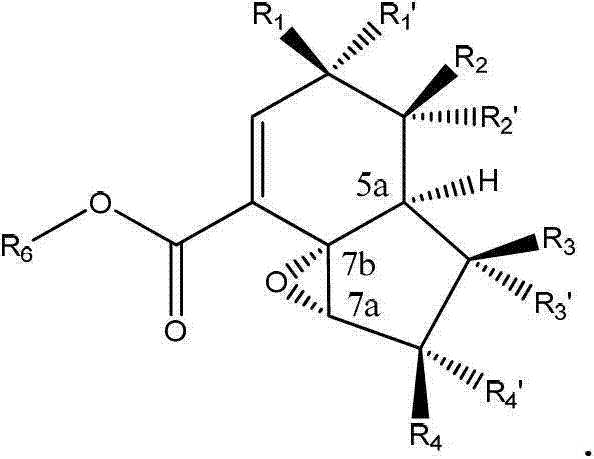
[0162] The final compound of formula (II) can be prepared via the electron enriched double bond of intermediate (III) using mCPBA, dimethyldioxirane, trifluoromethyldioxirane, peracid or similar obtained by regioselective and stereoselective epoxidation.
[0163] Process for the preparation of final compounds of formula (I) by lactonization (Scheme 2)
[0164] Methods of preparation of final compounds of formula (I) by lactonization (Scheme2)
[0165]
[0166] Scheme 2: Example of a non-limiting method for the preparation of the final compound of formula (I) as disclosed herein by hydrolysis and lactonization of the compound of formula (II) as disclosed herein.
[0167] Ester hydrolysis of (II) under basic or acidic conditions, followed by acid-catalyzed epoxide opening and subsequent lactonization yields the final lactone compound (I).
[0168] Preparation of tetrahydroindene intermediates of formula (III) via intermolecular [4+2]vinyl allene Diels-Alder cyclization ...
Embodiment 1
[0227] 2a1-Hydroxy-4,7,7-trimethyl-4,5,5a,6,7,7a-hexahydroindeno[1,7-bc]furan-2(2a1H)-one
[0228]
[0229] 1 H NMR (CDCl 3 )6.91(1H,d,J=3.8Hz),4.30(1H,s),2.49(1H,m),2.43(1H,m),2.11(1H,m),1.87(1H,ddd,J=14.6 , 6.5 and 2.4Hz), 1.63 (1H, dd, J = 10.0 and 4.2Hz), 1.57 (1H, dd, J = 10.2 and 4.1Hz), 1.45 (1H, dd, J = 13.2 and 6.9Hz), 1.22 (3H,d,J=7.2Hz),1.17(3H,s),0.91(3H,s)
[0230] 13 C NMR (CDCl 3 ) 170.1, 146.8, 128.7, 107.2, 95.3, 80.5, 41.9, 39.9, 39.1, 30.0, 29.3, 28.0, 24.7, 19.8
[0231] HRMS C 13 h 19 o 3 [M+1] Calculated: 223.1334, Measured: 223.1345
Embodiment 2
[0233] 2a 1 -Hydroxy-4-methyl-7-phenyl-4,5,5a,6,7,7a-hexahydroindeno[1,7-bc]furan-2(2a 1 H)-keto
[0234]
[0235] 1 H NMR (CDCl 3 )7.31(2H,m),7.22(1H,m),7.06(2H,d,J=7.3Hz),7.01(1H,d,J=3.9Hz),4.93(1H,d,J=8.5Hz) ,3.53(1H,dt,J=12.5 and 7.9Hz),2.56(1H,m),2.50(1H,m),2.00(2H,m),1.73(1H,ddd,J=14.3,10.0 and 4.3Hz ),1.60(1H,q,J=12.5Hz),1.27(3H,d,J=7.2Hz)
[0236] 13 C NMR (CDCl 3 ) 170.1, 147.2, 137.1, 128.9, 128.8, 128.6, 127.2, 88.9, 82.8, 80.1, 47.4, 41.1, 33.7, 29.6, 27.9, 19.9
[0237] HRMS C 17 h 18 o 3 Na[M+1] Calculated: 293.1154, Measured: 293.1147
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com