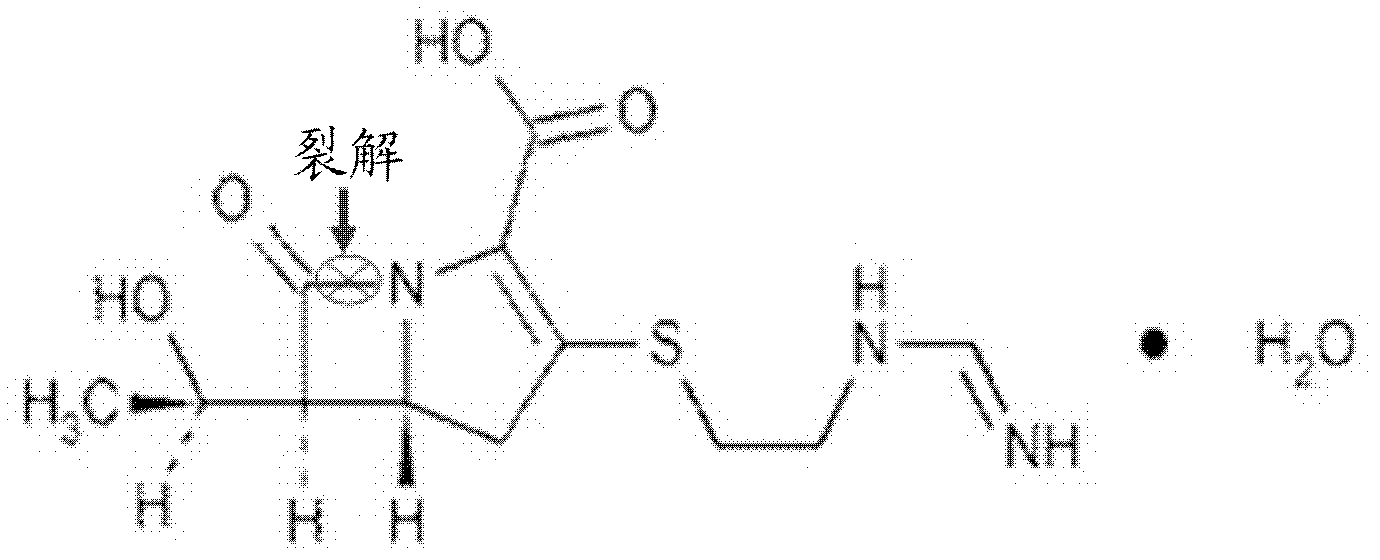
Purpose of L-cysteine compound for restraining new delhi metallo (NDM)-1 activity
A technology of cysteine and compounds, which is applied in the field of pharmacy, can solve the problems of undiscovered L-cysteine compounds and achieve the effects of treating drug-resistant bacterial infections, eliminating hydrolysis, and good drug application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
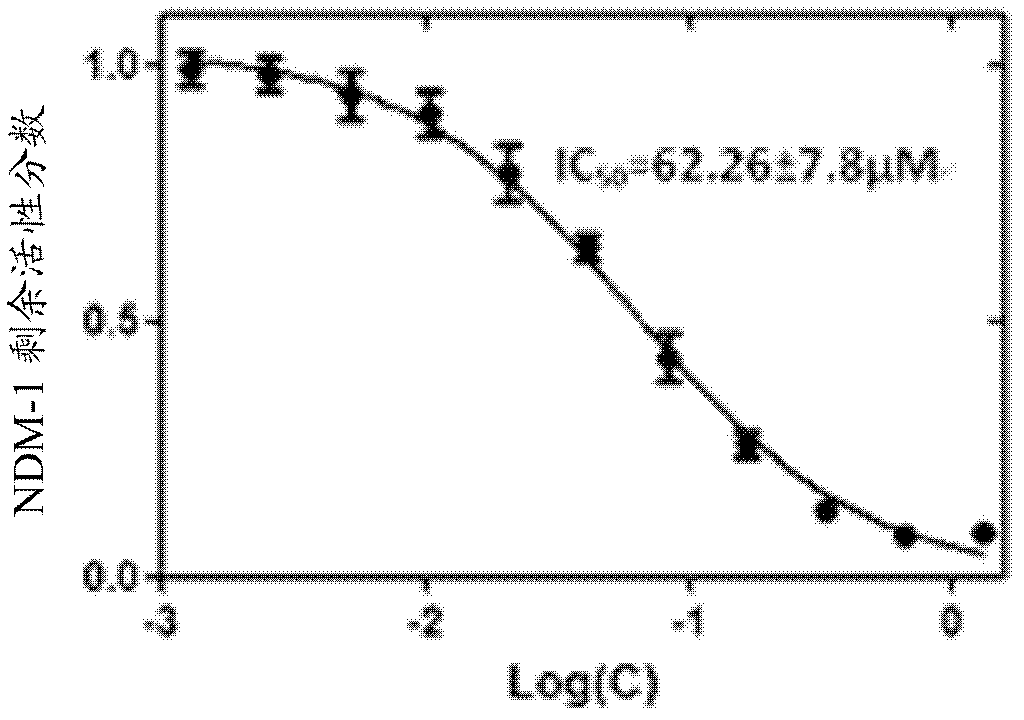
[0052] Example 1 Determination of NDM-1 activity inhibited by N-benzyloxycarbonyl-L-cysteine
[0053]
[0054] The N-benzyloxycarbonyl-L-cysteine used was purchased from Bailingwei with a purity of 98%.
[0055] Dissolve N-benzyloxycarbonyl-L-cysteine (4 mg) in 95% DMSO double-distilled water (156.7 μL) to prepare a solution with a concentration of 100 mM, then place the solution in a 1.5 ml ep tube, and Store at 4°C.
[0056] Then according to the above activity test method step 5 (preliminary screening of compound) and step 6 (IC of compound) 50 Determination of value) to carry out the operation, then take the concentration logarithm of N-benzyloxycarbonyl-L-cysteine as the abscissa, and the residual activity fraction of NDM-1 as the ordinate to draw a curve, see image 3 . Finally, according to the curve, using GraphPad Prism version 5.0 software to calculate, the obtained IC 50 The value was 62.26±7.8 μM.
Embodiment 2
[0057] Example 2 Determination of NDM-1 Activity Inhibited by N-acetyl-L-cysteine
[0058]
[0059] The N-acetyl-L-cysteine used was purchased from Bailingwei with a purity of 99%.
[0060] N-acetyl-L-cysteine (4 mg) was dissolved in 95% DMSO double distilled water (245.1 μL) to prepare a solution with a concentration of 100 mM, and then the solution was placed in a 1.5 ml ep tube, at 4 Store at ℃.
[0061] Then operate according to the content described in step 5 and step 6 of the above activity test method, and then take the logarithm of the concentration of N-acetyl-L-cysteine as the abscissa, and the remaining activity fraction of NDM-1 as the ordinate To draw a curve, see Figure 4 . Finally, according to the curve, using GraphPad Prism version5.0 software to calculate, the obtained IC 50 The value was 195.1±35 μM.
Embodiment 3
[0062] Example 3 Determination of NDM-1 activity inhibited by N-tert-butoxycarbonyl-L-cysteine
[0063]
[0064] The N-tert-butoxycarbonyl-L-cysteine used has a purity of 98%.
[0065] Dissolve N-tert-butoxycarbonyl-L-cysteine (4mg) in 95% DMSO (180.8μL) to prepare a solution with a concentration of 100mM, then place the solution in a 1.5ml ep tube, and store at 4°C Save it.
[0066] Then operate according to the content described in the above activity test method step 5 and step 6, then take the logarithm of the concentration of N-tert-butoxycarbonyl-L-cysteine as the abscissa, and the residual activity fraction of NDM-1 is To draw a curve on the ordinate, see Figure 5 . Finally, according to the curve, the GraphPadPrism version 5.0 software is used to calculate the obtained IC 50 The value was 404.3±49.7 μM.
[0067] According to the above examples, it can be known that the compounds involved in the present invention can inhibit the activity of NDM-1, and can ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com