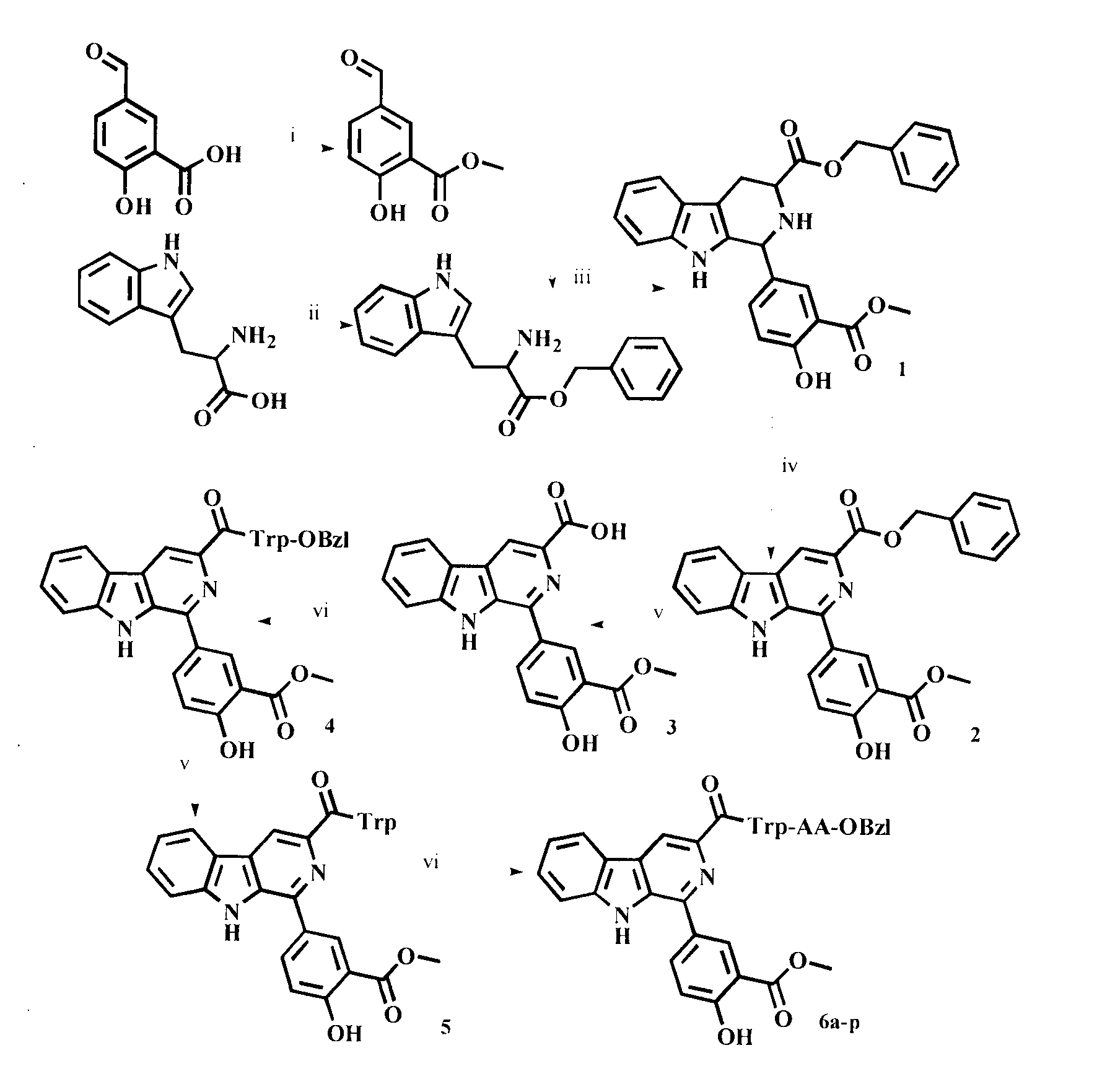
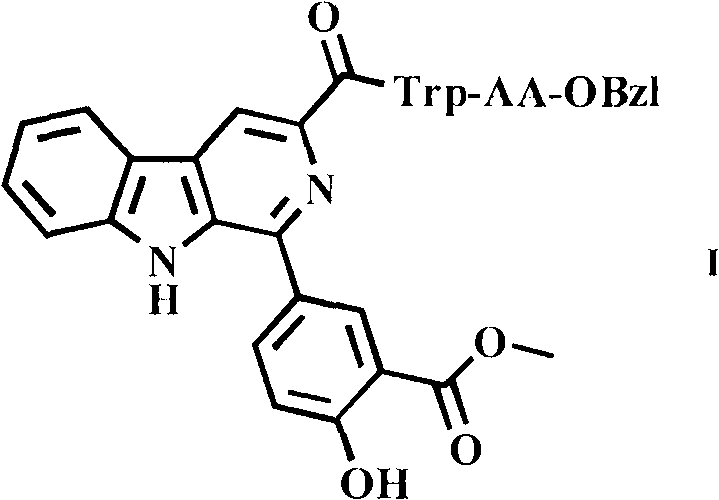
1-(4-hydroxy-3-methoxycarbonyl)-beta-carboline-3-formyl tryptophyl amino acid benzyl ester, and synthesis and application thereof
A technology of tryptophan benzyl ester and methoxycarbonyl, which is applied in the field of biomedicine and can solve problems such as no obvious progress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1 prepares methyl 5-formylsalicylate
[0020] Weigh 1.660g (10.0mmol) of 5-formylsalicylic acid in a microwave reaction tank, add 25mL of methanol and 1mL of concentrated H 2 SO 4 , reacted in a microwave reactor at 90°C for 2 h, monitored by TLC until the raw material spots disappeared, stopped the reaction and cooled down to room temperature, transferred the reaction mixture to a 100 mL eggplant-shaped bottle, adjusted the pH value to 8 with concentrated ammonia water, and reduced the reaction mixture to Concentrate to dryness under reduced pressure, and add a large amount of ethyl acetate to dissolve the residue. The ethyl acetate layer was sequentially washed with saturated NaHCO 3 solution and saturated NaCl solution were washed three times each, and then washed with anhydrous NaCl 2 SO 4 Dry for 2 hours, filter, and concentrate the filtrate to dryness under reduced pressure. After standing at room temperature overnight, crystals precipitate out to o...
Embodiment 2
[0021] Embodiment 2 prepares L-tryptophan benzyl ester
[0022] Weigh 15.0g (44.4mmol) of polyphosphoric acid in a 500mL eggplant-shaped bottle, add 80mL of benzyl alcohol, and dissolve it in an oil bath at 50°C. After the temperature of the solution rises to 75°C, weigh 10g (49.0mmol) of L - Add tryptophan to it, react at 75°C for 48 hours, use TLC to monitor until the raw material spots disappear, stop the reaction and cool down, add 400mL of anhydrous ether to the reaction bottle under stirring in an ice bath, at this time, a colorless solid precipitates, stir After overnight filtration, the colorless solid was suspended with 200mL ethyl acetate and 10mL water, and the pH value of the solution was adjusted to about 8 with triethylamine. 3 solution and saturated NaCl solution were washed three times each. Ethyl acetate layer with anhydrous Na 2 SO 4 It was dried for 2 h, filtered, and the filtrate was concentrated under reduced pressure to obtain 12.85 g (89.2%) of the ti...
Embodiment 3
[0023] Example 3 Preparation of 1-(4-hydroxyl-3-methoxycarbonyl)-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid benzyl ester (1)
[0024] Add 100mL CH to a 250mL eggplant-shaped bottle 2 Cl 2 and 10mL TFA, after stirring evenly, weigh 11.76g (40.0mmol) L-tryptophan benzyl ester and 7.92g (44.0mmol) methyl 5-formylsalicylate and add it, and the reaction solution turns reddish after a few minutes After 2 days, the reaction solution turned black. Slowly add concentrated ammonia water dropwise under stirring in an ice bath to adjust the pH value of the reaction solution to 8. The reaction mixture was allowed to stand for liquid separation, and the separated CH 2 Cl 2 Layers were sequentially washed with saturated NaHCO 3 solution and saturated NaCl solution were washed three times, CH 2 Cl 2 Anhydrous Na 2 SO 4 Dry for 2 h, filter, and concentrate the filtrate to dryness under alkaline pressure to obtain 14.59 g (80%) of the title compound as a yellow solid. ESI-MS(m / e):...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com