Method for producing nano basic copper nitrate
A production method and technology of copper nitrate, applied in directions such as copper nitrate, nanotechnology, nanotechnology, etc., can solve the problems of complex post-processing process, high difficulty in industrial production, low yield, etc., and achieve elimination of application and promotion defects, combustion And the effect of good catalytic performance and low equipment cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Prepare the following reaction solutions with deionized water or double distilled water: copper nitrate aqueous solution, caustic soda solution and ammonium nitrate solution, and carry out ultrafiltration treatment to copper nitrate aqueous solution and caustic soda solution; the mass concentration of described copper nitrate solution is 48~52%, and temperature is 25~28 ℃; The mass concentration of described caustic soda solution is 23~27%, and temperature is 25~28 ℃; The mass concentration of described ammonium nitrate solution is 1.0~1.4%, and temperature is 25~28 ℃; 28℃, pH value is 3.4~3.6;
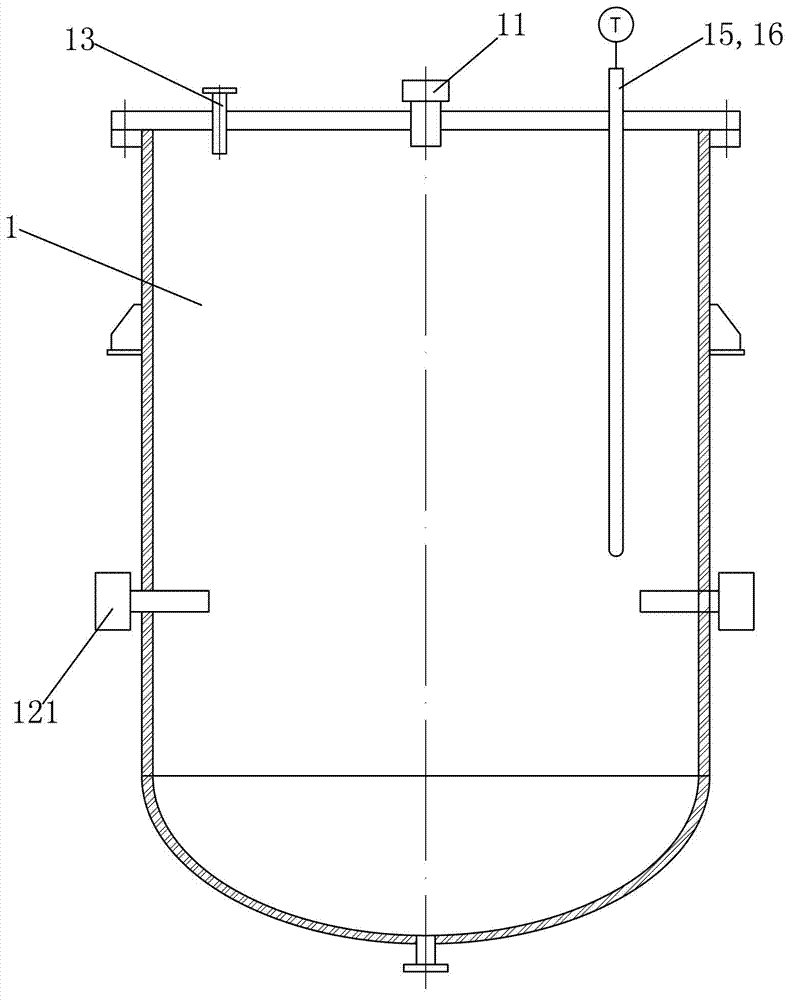
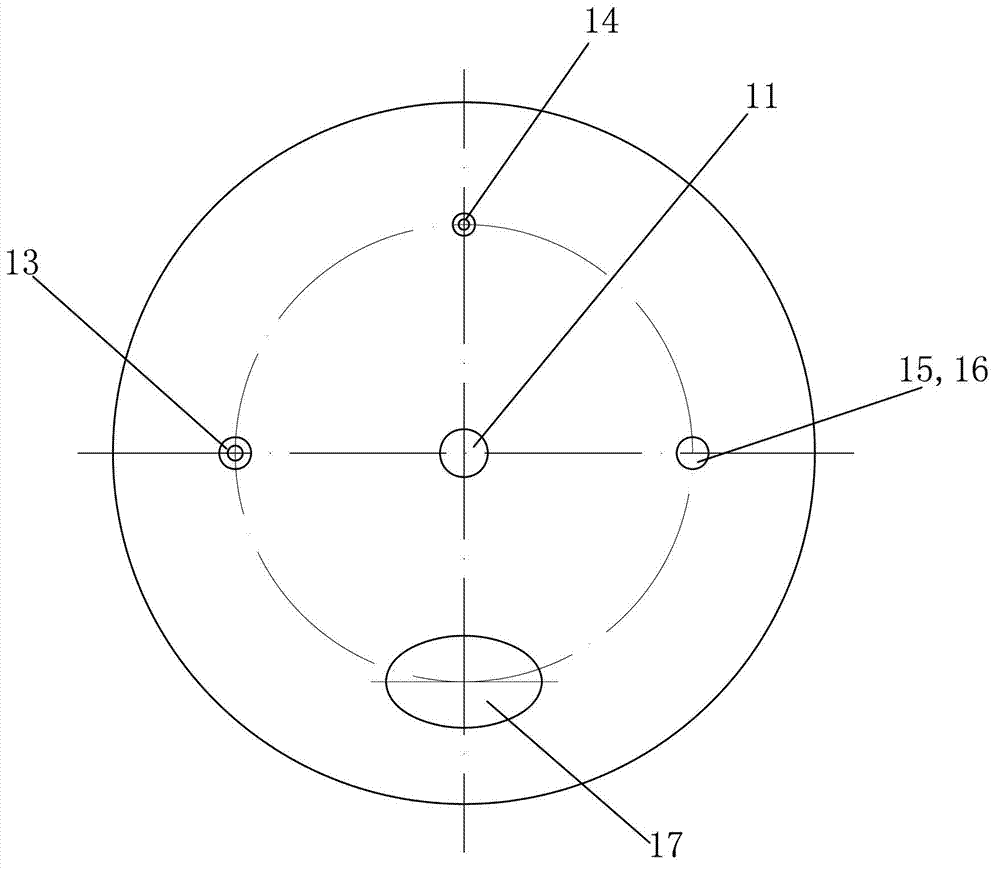
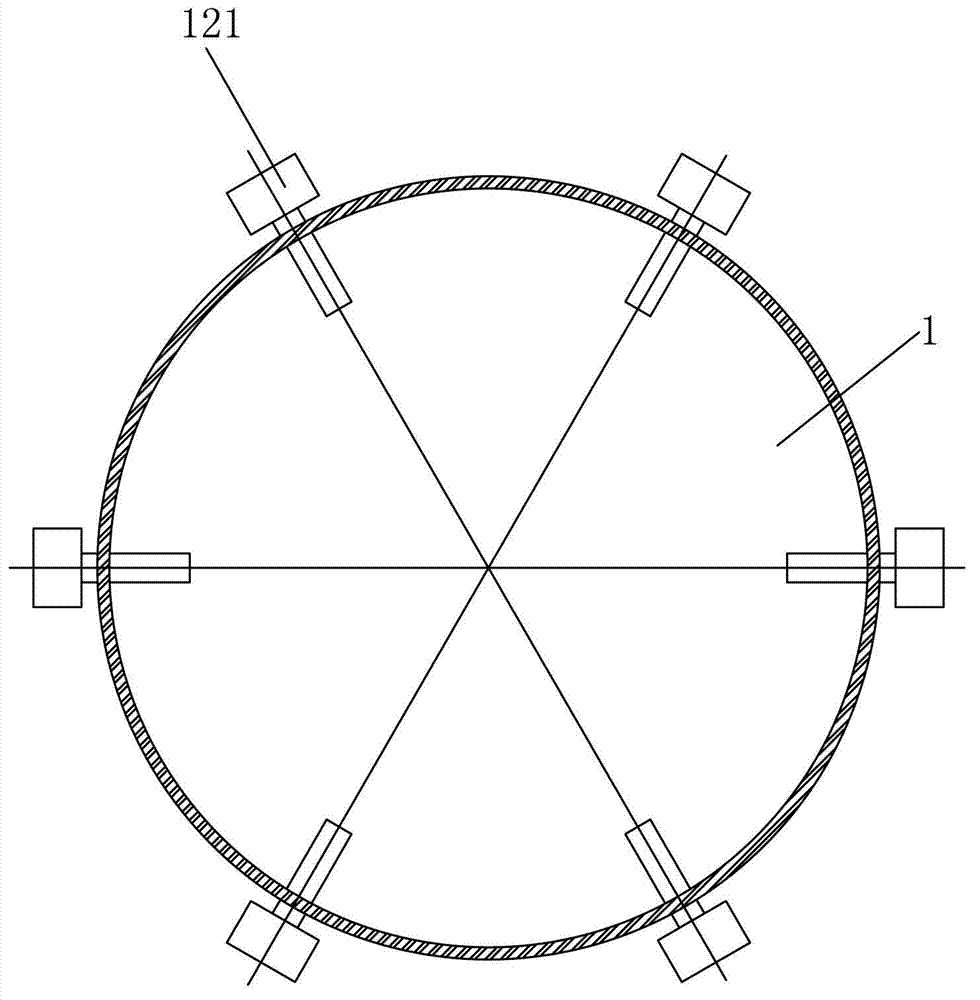
[0031] (2) Put the ammonium nitrate solution in such as Figures 1 to 3 In the shown reactor equipped with an ultrasonic stirring device, the ultrafiltered copper nitrate solution and caustic soda solution (volume ratio 1:1) are passed through as follows: Figure 4 The mixed liquid high-pressure spray device shown is sent into the above-mentioned reactor for reaction, that...
Embodiment 2
[0038] (1) Prepare the following reaction solution with deionized water or double distilled water: strontium carbonate aqueous solution, caustic soda solution and ammonium nitrate solution, and carry out ultrafiltration treatment to strontium carbonate aqueous solution and caustic soda solution; the mass concentration of described strontium carbonate solution is 48~52%, and temperature is 25~28 ℃; The mass concentration of described caustic soda solution is 23~27%, and temperature is 25~28 ℃; The mass concentration of described ammonium nitrate solution is 1.0~1.4%, and temperature is 25~28 ℃; 28℃, pH value is 3.4~3.6;
[0039] (2) Put the ammonium nitrate solution in such as Figures 1 to 3 In the shown reactor equipped with an ultrasonic stirring device, the ultrafiltered strontium carbonate solution and caustic soda solution (volume ratio 1:1) are passed through as follows: Figure 4 The mixed liquid high-pressure spray device shown is sent into the above-mentioned reactor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com