Method for synthesis of phenylacetic acid by carbonylation of benzyl chloride
A benzyl chlorocarbonyl compound synthesis technology, applied in chemical instruments and methods, carbon monoxide reaction to prepare carboxylic acid, organic compound/hydride/coordination complex catalyst, etc., can solve the problem of complex catalyst preparation, limitation of phenylacetic acid promotion, and difficult Long-term storage and other issues, to achieve excellent catalytic activity, easy treatment and purification, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Synthesis of 2-(4-amino-2-phenylhydroxy)benzimidazole
[0021] Add 0.12 mol of o-phenylenediamine, 0.1 mol of p-aminosalicylic acid and 100.0 ml of polyphosphoric acid into a 150 ml round bottom flask, and react with mechanical stirring at 190°C for 4 hours. After the reaction is over, cool the reaction solution to 100°C and pour it into ice water. After filtering, washing and recrystallizing the precipitated precipitate, the obtained yellow powder is 2-(4-amino-2-phenylhydroxy)benzimidazole. The yield was 86%.
[0022] 2-(4-Amino-2-phenylhydroxy)benzimidazole C 13 h 11 N 3 O: m.p. 243–244 ° C (lit. 241–242 ° C). 1 H NMR (DMSO- d 6 , 400 MHz), δ (ppm): 12.98 (s, 1H, OH), 12.67 (s, 1H, NH), 7.66 (d, J = 8.5 Hz, 1H), 7.52 (s, 2H), 7.18 (dd, J = 5.7, 3.0 Hz, 2H), 6.21 (d, J = 8.5 Hz, 1H), 6.14 (s, 1H), 5.67 (s, 2H, NH 2 ).IR (KBr), ν (cm -1 ): 3437, 3353, 3331, 3235, 1637, 1591, 1484, 1412, 1268, 964, 899, 842, 805, 745. MS m / z (%): 226 ...
Embodiment 2
[0023] Embodiment 2: the synthesis of transition metal complex
[0024] In a 25ml round bottom flask, add 0.5 mmol CoCl 2 ·6H 2 O. 1.2 mmol 2-(4-amino-2-phenylhydroxy)benzimidazole, 10 ml methanol as solvent, stir at room temperature for 4-5 hours, a maroon precipitate forms, filter, wash the solid with a small amount of methanol, and let it dry naturally at room temperature Dry.
[0025] IR (KBr), ν (cm -1 ): 3418, 1618, 1494, 1393, 1262, 1131, 817, 743, 617, 519, 466 cm -1 ; Elemental analysis (theoretical): C: 62.18 (61.54), H: 3.07 (3.97), N: 15.91 (16.56).
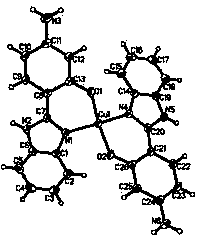
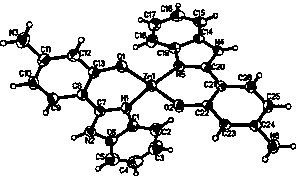
[0026] The synthesis method of Cu and Zn metal complexes is similar to the above-mentioned method, and a suitable single crystal is cultivated through recrystallization, and the structure is as follows: figure 1 and 2 shown.
[0027] 2-(4-Amino-2-phenylhydroxy)benzimidazole copper complex Cu(C 13 h 10 N 3 O) 2 : IR (KBr), ν (cm -1 ): 3375, 3000, 1610, 1500, 1440, 1250, 1125, 930, 745, 513, 470. Elemen...
Embodiment 3
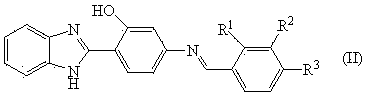
[0029] Embodiment 3: the synthesis of catalytic promoter benzimidazole Schiff base
[0030] In a 50 ml round bottom flask, add 2.5 mmol 2,4-dichlorobenzaldehyde, 2 mmol 2-(4-amino-2-phenylhydroxy)benzimidazole and 10.0 ml anhydrous methanol, stir and reflux at 65 °C 4h. After the reaction solution was cooled and left to stand, light yellow powder precipitated out. After filtration, washing and recrystallization, the Schiff base was obtained with a yield of 73%.
[0031] The Schiff base C obtained above 20 h 13 Cl 2 N 3 O: m.p. 300–301 ° c. 1 H NMR (DMSO- d 6, 400 MHz), δ (ppm):13.33 (s, 2H), 8.99 (s, 1H), 8.20 (d, J = 10.5 Hz, 2H), 7.82 (s, 1H), 7.69 (s, 2H), 7.60 (d, J = 7.8 Hz, 1H), 7.30 (dd, J = 5.4, 2.8 Hz, 3H), 7.12 (d, J = 8.7 Hz, 1H). IR (KBr), ν (cm -1 ): 3321, 3059, 1583, 1495, 1387, 1262, 1132, 869, 811, 744. MS: m / z (%): 382 (M+1). Anal. calcd. for C 20 h 13 Cl 2 N 3 O: C 62.18, H 3.13, N 10.13; Found: C 62.84, H 3.43, N 10.99.
[0032]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com