Etimicin sulfate pseudo-polymorph, and preparation method and application thereof
A pseudopolymorphic, sulfate technology, applied in the preparation of sugar derivatives, chemical instruments and methods, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
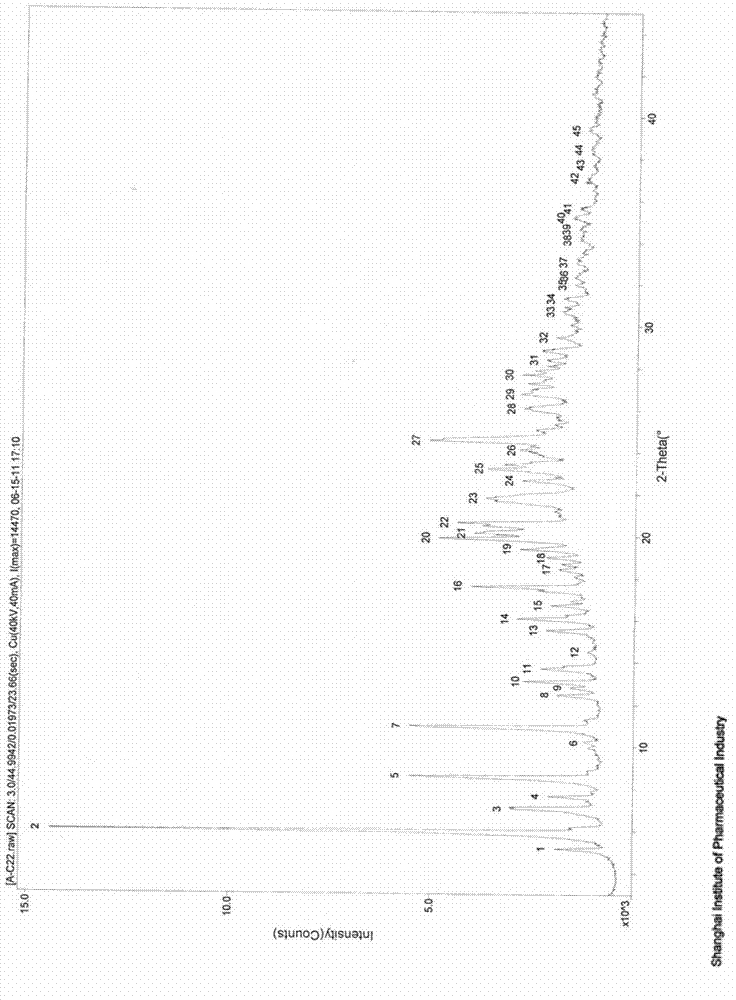
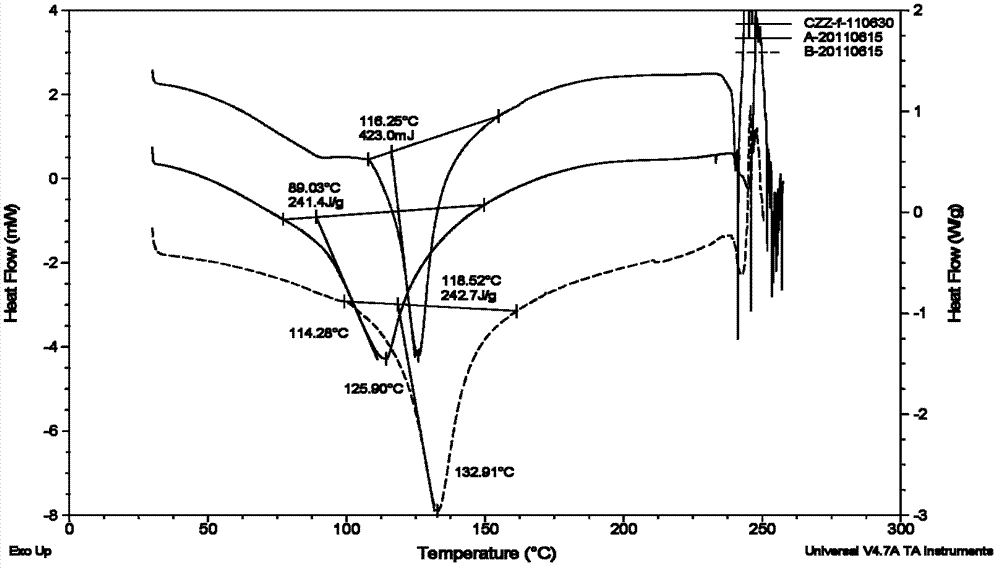
[0038] 2 g of 1-N-ethyl gentamicin C 1a Sulfate was dissolved in 10ml of water and added to the kettle. Then 30ml of methanol was added to the reaction kettle, and the temperature was maintained at 40°C under stirring. And add 4 mol / L aqueous sulfuric acid solution dropwise to the reactor to adjust the final pH of the mother liquor to 5.0. After stirring for 1 h, it was filtered to obtain 1.3 g of dry crystalline solid. The dried product was identified as pseudopolymorph I by X-ray powder diffraction, DSC spectrum and TGA spectrum. The polymorph I is shown to have 2θ° of about 5.1, 6.0, 7.0, 7.6, 8.5, 10.9, 12.5, 13.1, 13.7, 15.5, 16.0, 16.6, 17.2, 17.6, 19.4, 19.9, 20.1, 20.5, 21.7, The X-ray powder diffraction patterns of the characteristic peaks represented by 22.6, 23.2, 24.0, 24.5, 26.0, 26.7, 27.1, 27.6, 28.4 and 28.8 are shown in Figure 1. The melting point of this pseudopolymorph I was measured to be 114°C-133°C.
Embodiment 2
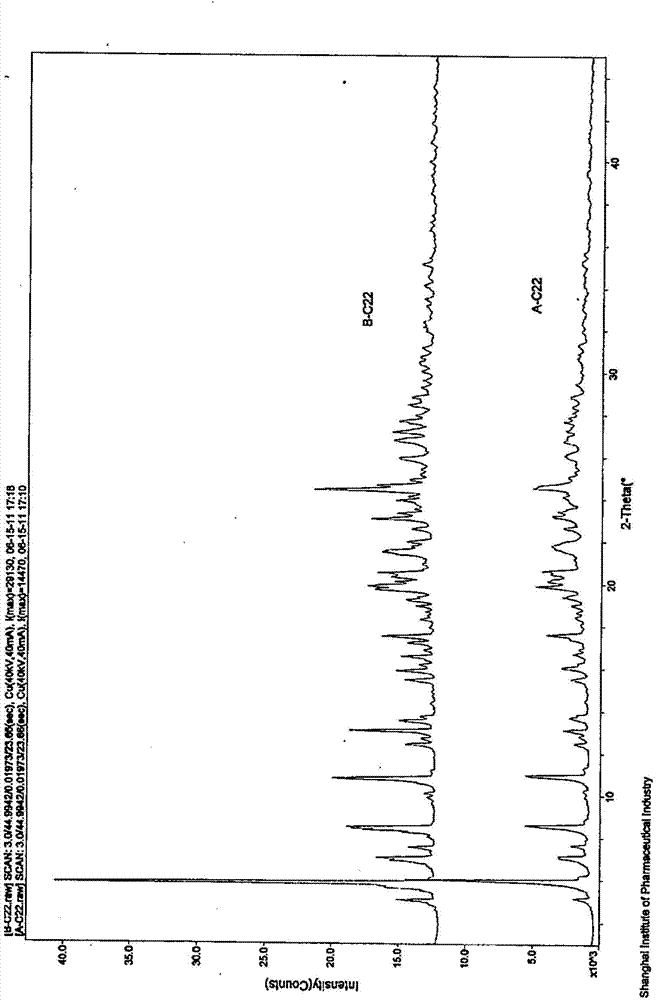
[0040] 6 g of 1-N-ethyl gentamicin C 1a Sulfate was dissolved in 10ml of water and added to the kettle. Then, 60 ml of methanol was added to the reaction kettle, and the temperature was maintained at 40° C. under stirring. And add dropwise 4mol / L aqueous sulfuric acid solution to the reactor to adjust the final pH of the mother liquor to 4.5. After stirring for 1 h, it was filtered to obtain 7.5 g of dry crystalline solid. The dry product was analyzed by X-ray powder diffraction ( Figure 1A ), DSC spectrum ( figure 2 upper solid line) and TGA spectrum ( image 3 ) was identified as pseudopolymorph I. The melting point of this pseudopolymorph I was measured to be 114°C-133°C.
Embodiment 3
[0042] 6 g of 1-N-ethyl gentamicin C 1a Sulfate was dissolved in 10ml of water and added to the kettle. Then 20ml of ethanol was added to the reaction kettle, and the temperature was maintained at 60°C under stirring. And add 6 mol / L aqueous sulfuric acid solution dropwise to the reactor to adjust the final pH of the mother liquor to 6.5. After stirring for 1 h, it was filtered to obtain 6.4 g of dry crystalline solid. Dried product uses X-ray powder diffraction, DSC collection of illustrative plates ( figure 2 The solid line in the middle) and the TGA pattern were identified as pseudopolymorph I. The melting point of this pseudopolymorph I was measured to be 114°C-133°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com