Method of determining risk of arrythmia
An arrhythmia, risk technology, used in biological testing, measurement devices, material inspection products, etc., can solve problems such as limiting the flow and duration of MEA research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
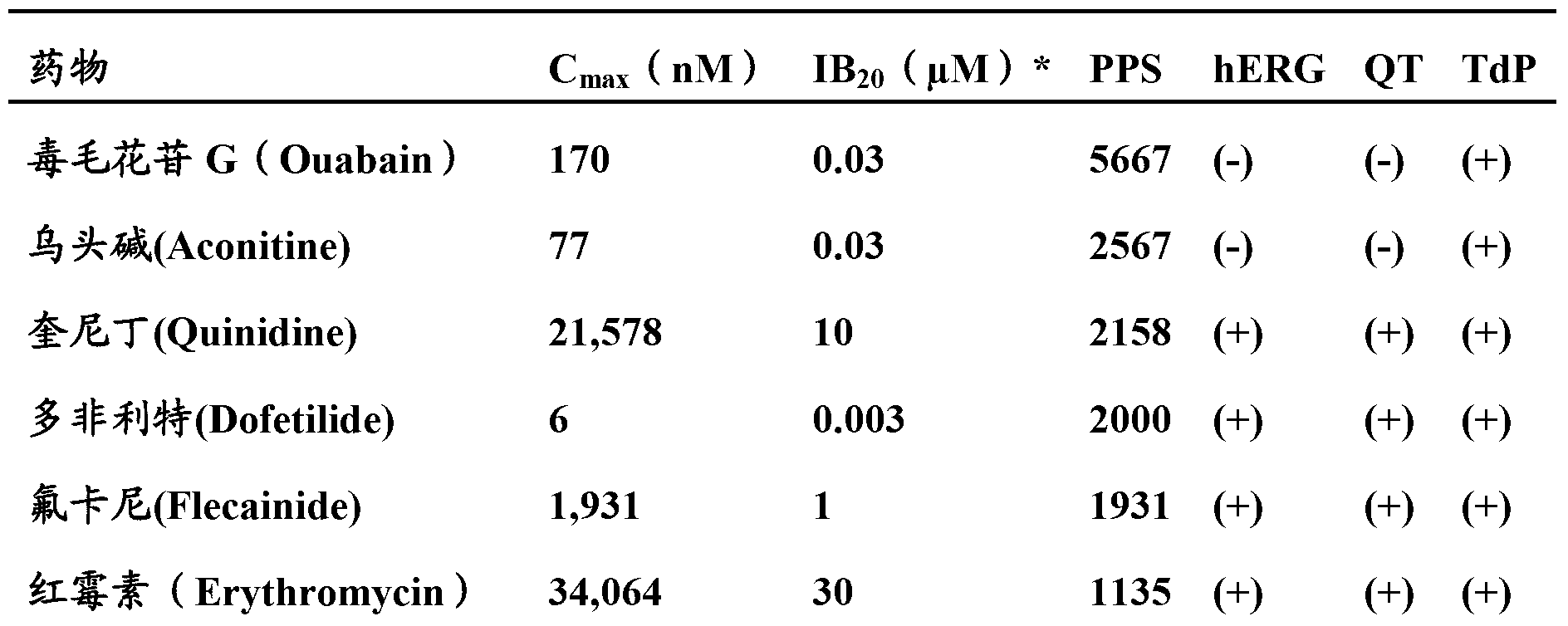
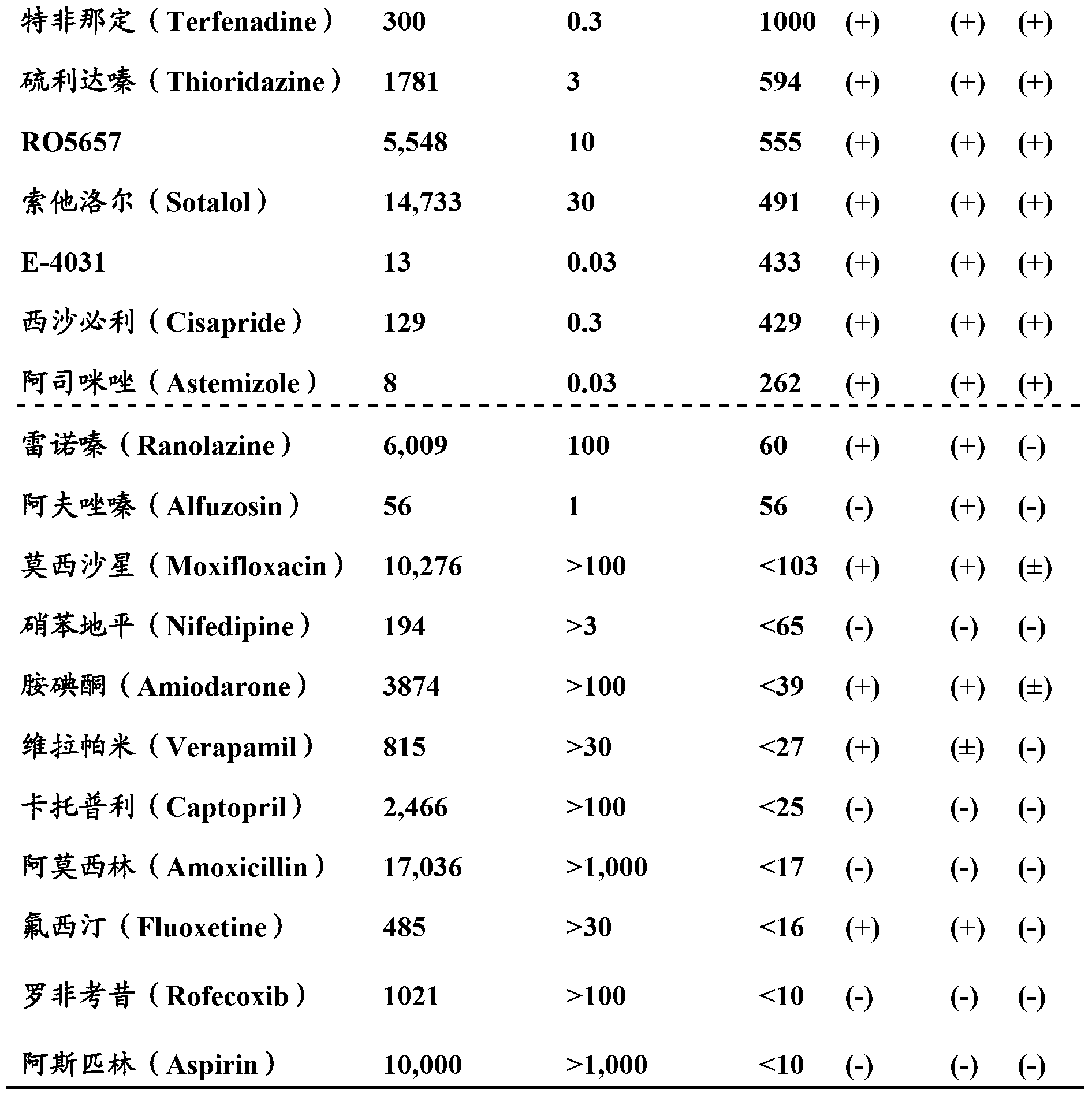
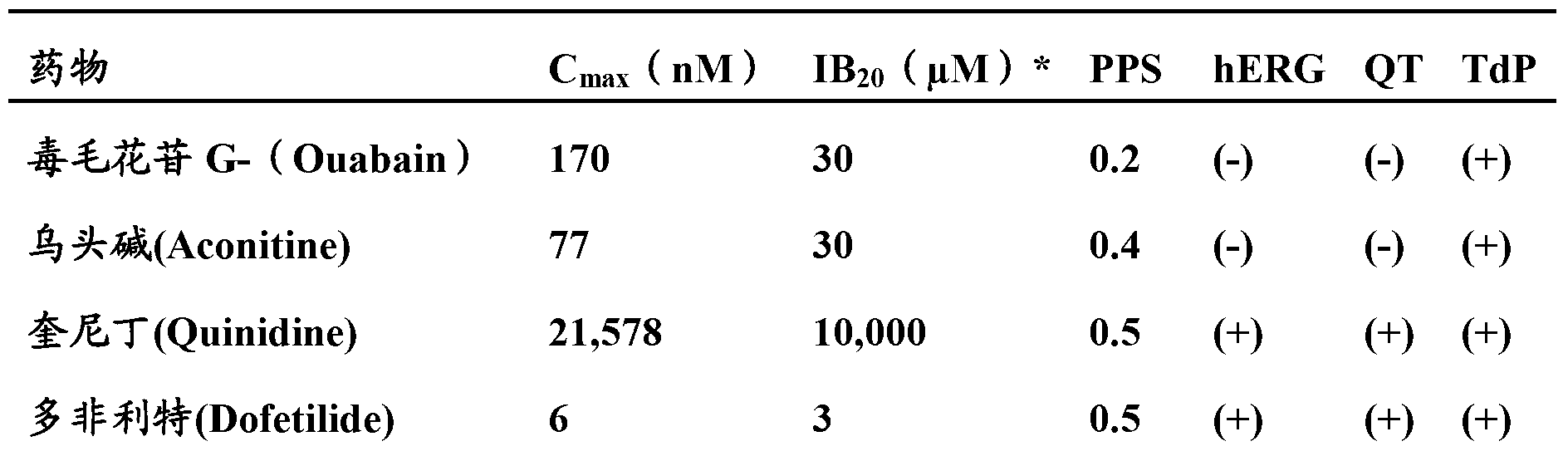
Image
Examples
Embodiment
[0092] method
[0093] Chemicals
[0094] In vitro reagents were purchased from Sigma Chemical Co. (St Louis MO).
[0095] method
[0096] cell culture. Thaw hiPSC-derived cardiomyocytes (iCells) from Cellular Dynamics International (CDI) in Plating Media (CDI) and grow at 2.2–2.7 × 10 per well 6 Densities of individual cells were plated as single cells on 0.1% gelatin (Sigma)-coated 6-well tissue culture plates (Corning). At 37°C, 7% CO 2 Cells were cultured for 3-5 days, after which they were re-plated onto 96-well cardio-e-plates (ACEA / Roche Applied Sciences) or microelectrode arrays (MEA, Multichannel Systems). After plating, the medium was changed every two days using Maintenance Media (CDI) (prewarmed to 37°C to minimize temperature shock to the cells).
[0097] overview
[0098] The new RTCA cardio system allows monitoring of cellular experiments over longer time frames and measures the contraction (beating) of cells in parallel. At each measurement time point...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com