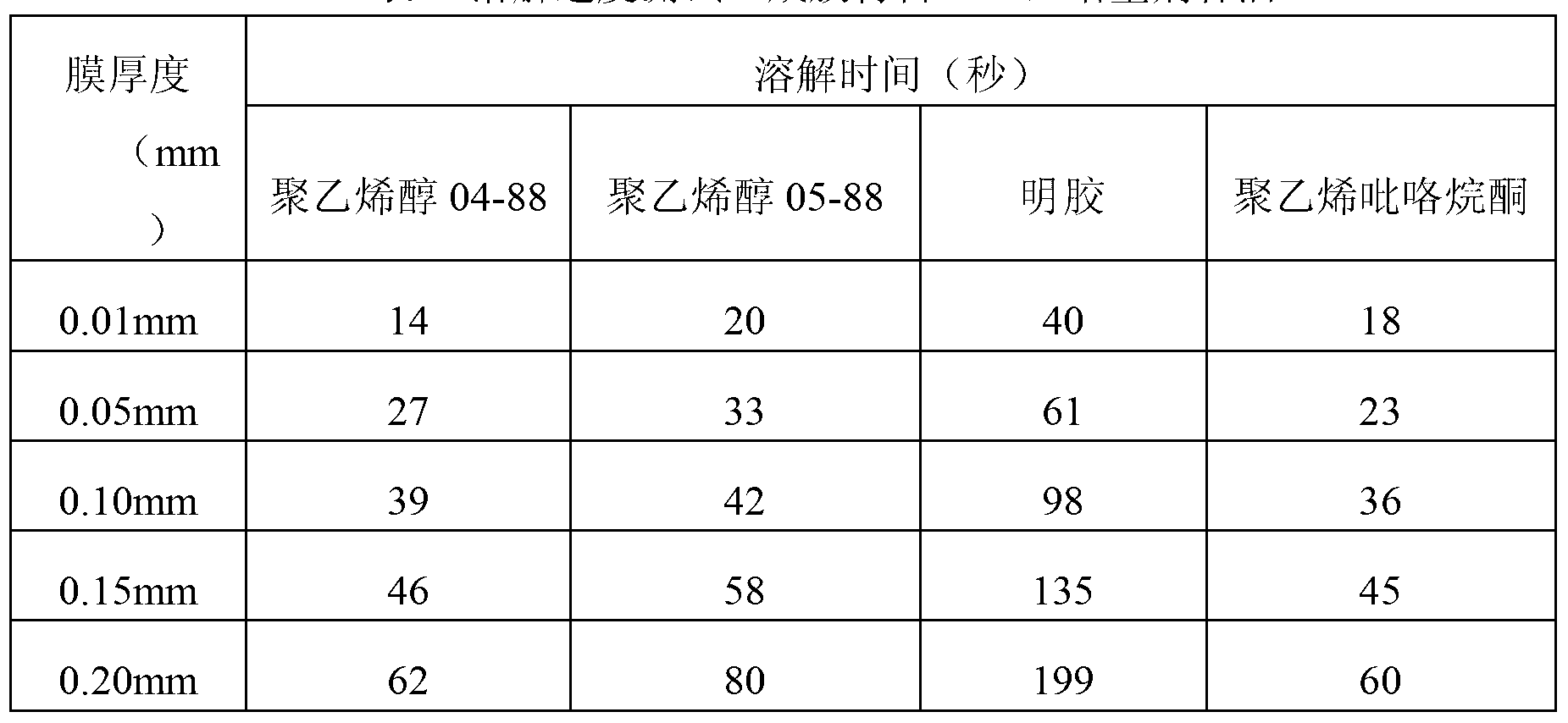
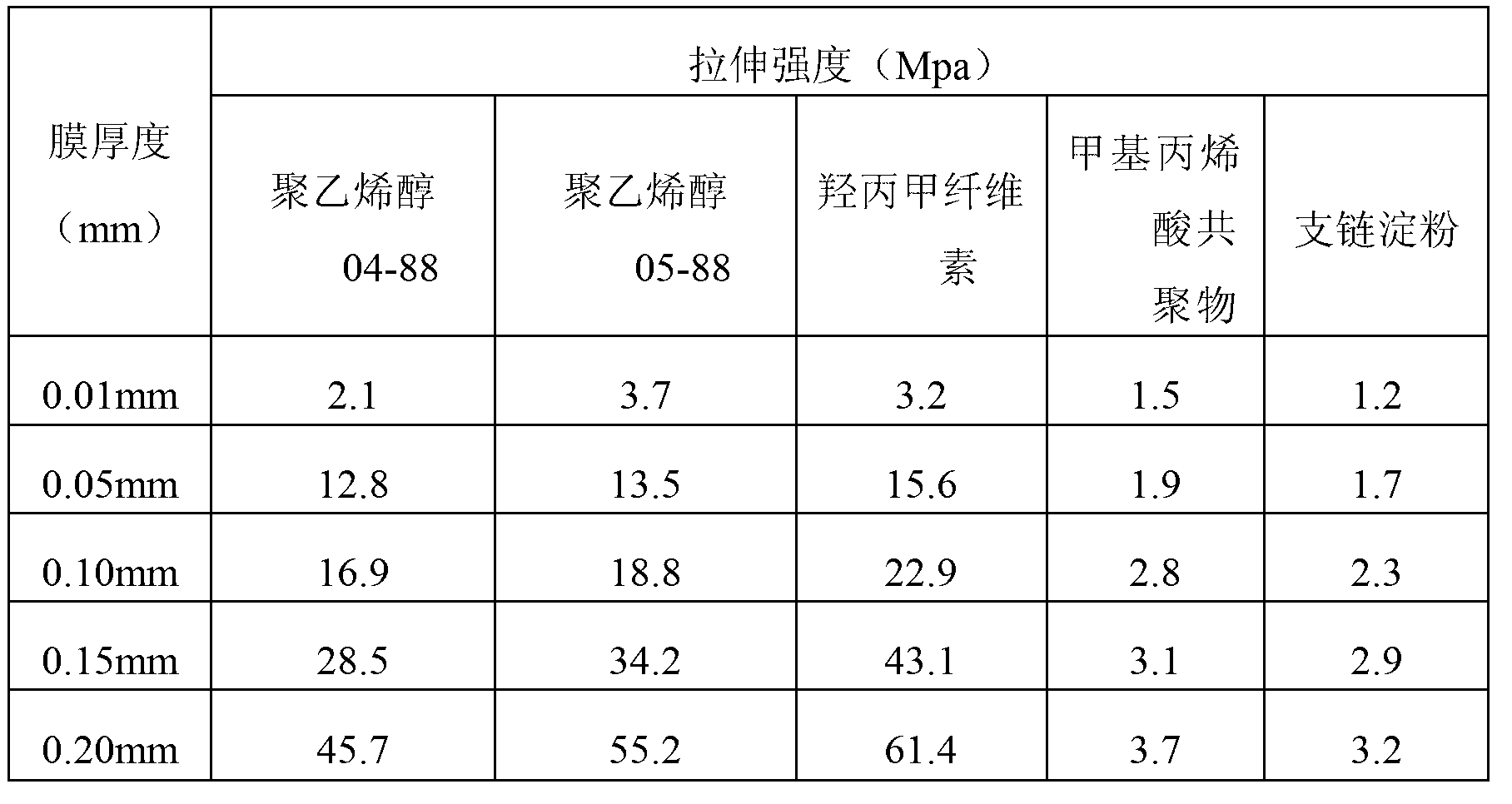
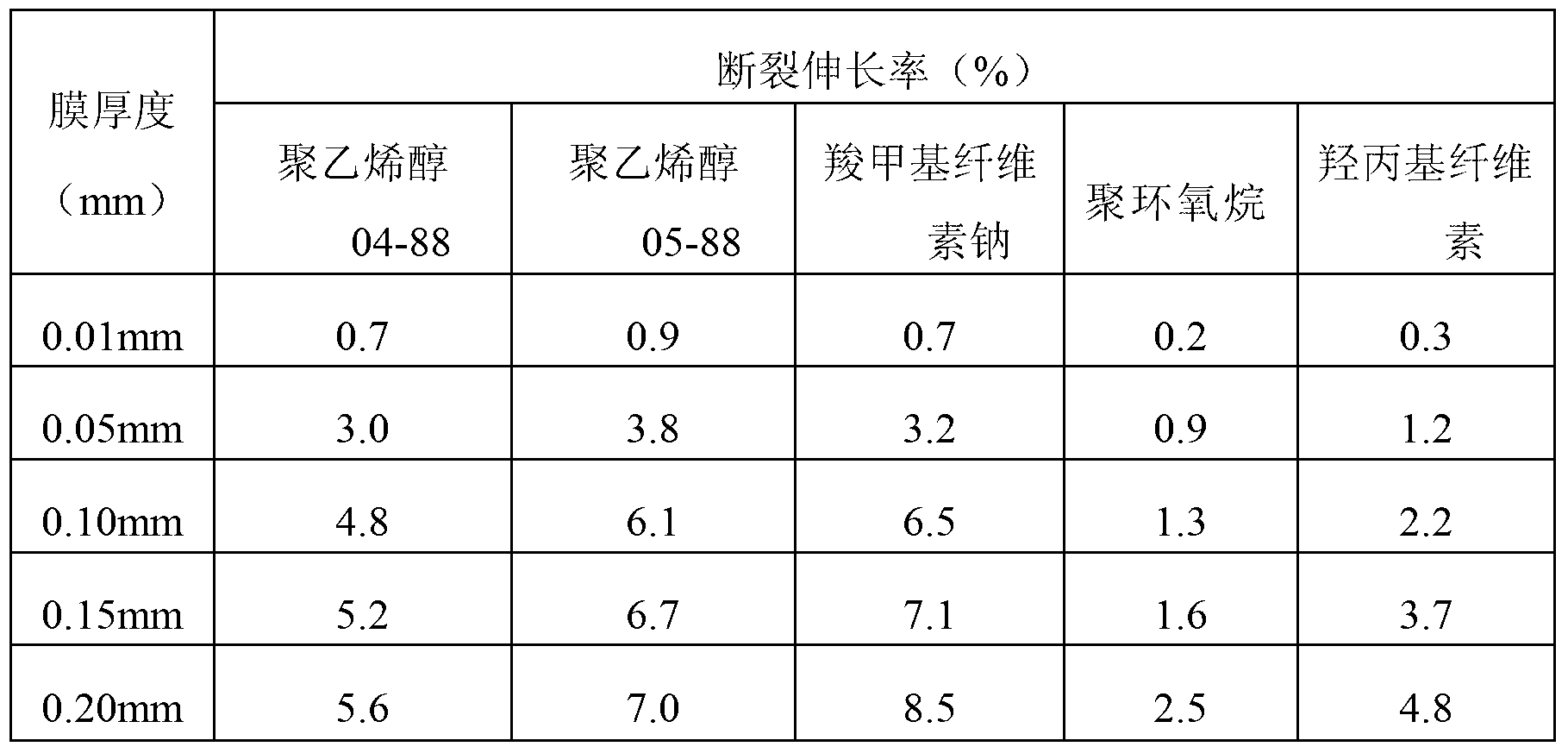
Method for testing sensitivity of pathogenic microorganisms to antibacterial drugs
A technology of pathogenic microorganisms and antibacterial drugs, which is applied in the field of medicine, can solve the problems of expensive consumables, inability to detect them, and inability to measure them with non-fermenting bacteria instruments, so as to save cumbersome operations and improve operational efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074]
[0075] Heat the purified water to 80°C, add polyvinyl alcohol 04-88, and stir to dissolve. Cool down to 25°C, add polyethylene glycol and amoxicillin, and stir for 0.5 hours. Stop stirring and let it stand for 4 hours to get rid of air bubbles. Films were applied and dried at 30°C. Remove the film, cut and pack. Sterilize.
Embodiment 2
[0077]
[0078] Heat the purified water to 80°C, add polyvinyl alcohol 04-88, and stir to dissolve. Cool down to 25°C, add polyethylene glycol and gentamicin sulfate, and stir for 0.5 hours. Stop stirring and let it stand for 4 hours to get rid of air bubbles. Films were applied and dried at 30°C. Remove the film, cut and pack. Sterilize.
Embodiment 3
[0080]
[0081] Heat the purified water to 80°C, add polyvinyl alcohol 04-88, and stir to dissolve. Cool down to 60°C, add polyethylene glycol and levofloxacin, and stir for 0.5 hours. Stop stirring and let it stand for 4 hours to get rid of air bubbles. Films were applied and dried at 60°C. Remove the film, cut and pack. Sterilize.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com