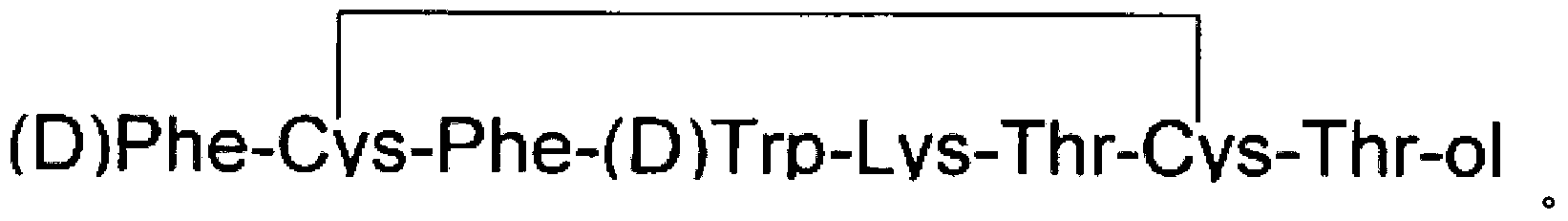Sustained release formulation comprising octreotide and two or more polylactide-co-glycolide polymers
A technology of octreotide and glycolide, which is applied in the field of sustained-release preparations, can solve problems such as fluctuations in plasma levels, and achieve the effect of reducing fluctuations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1: Preparation of microparticles
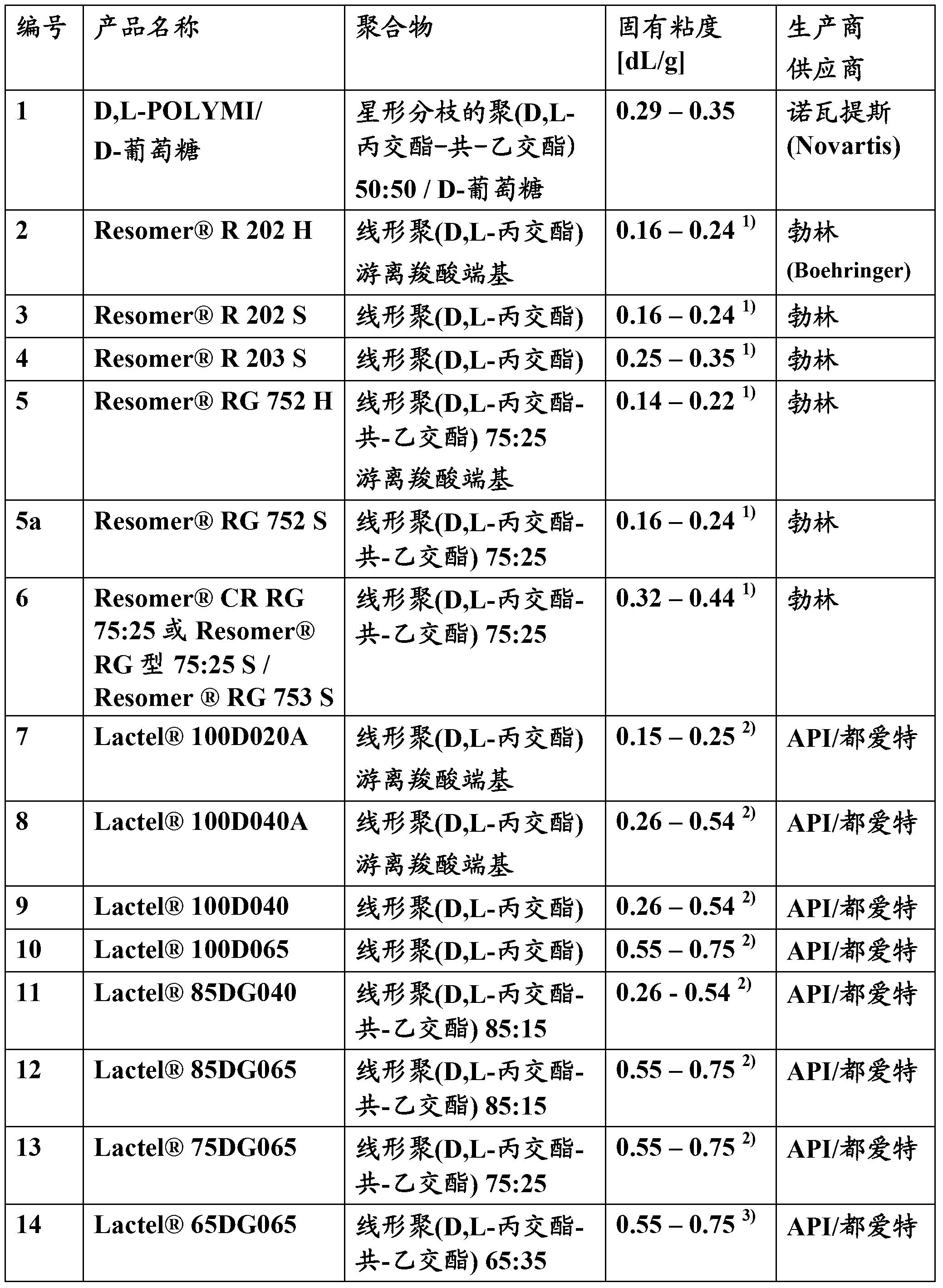
[0059] An appropriate amount of PLGA polymer was dissolved in an appropriate amount of methylene chloride to yield an appropriate polymer concentration as specified in Table 2 in the "PLGA Concentration" column. An appropriate amount of drug is weighed, placed in a glass beaker, and the polymer solution is poured over the drug so that the resulting microparticles have a drug loading as specified in the "Drug Loading" column.
[0060] For example for microparticles with a drug loading of 20% and a polymer concentration of 20%, the quantities are as follows: 3.547g of PLGA polymer was dissolved in 17.7ml of dichloromethane, resulting in a 20% (w / v) polymer solution. Weigh 1.453g of octreotide pamoate (equivalent to 1.00g=20% octreotide free base), place it in a glass beaker, and pour the polymer solution on the drug.
[0061] The suspension was homogenized with an Ultra-Turrax rotor-stator stirrer at 20'000 rpm for 1 minute wh...
Embodiment 2
[0087] Example 2: Carrier Compositions A to G
[0088] Under vigorous stirring with a magnetic stirrer, CMC-Na, Mannitol and Pluronic F68 in the amounts as given in Table 3 were dissolved in about 15 ml of hot deionized water at a temperature of about 90°C. The resulting clear solution was cooled to 20 °C and made up to 20.0 ml with deionized water.
[0089] Table 3: Carriers suitable for microparticles (amounts given in g)
[0090]
Embodiment 3
[0091] Example 3: Microparticle Suspension
[0092] 170 mg of the microparticles of Examples 1-33 were suspended in 1.0 ml of vehicle of composition D (Table 3) in a 6R vial. The suspension was homogenized by hand shaking for about 30 seconds. The reconstituted suspension could be injected without any problem using a 20 gauge needle.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Intrinsic viscosity | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com