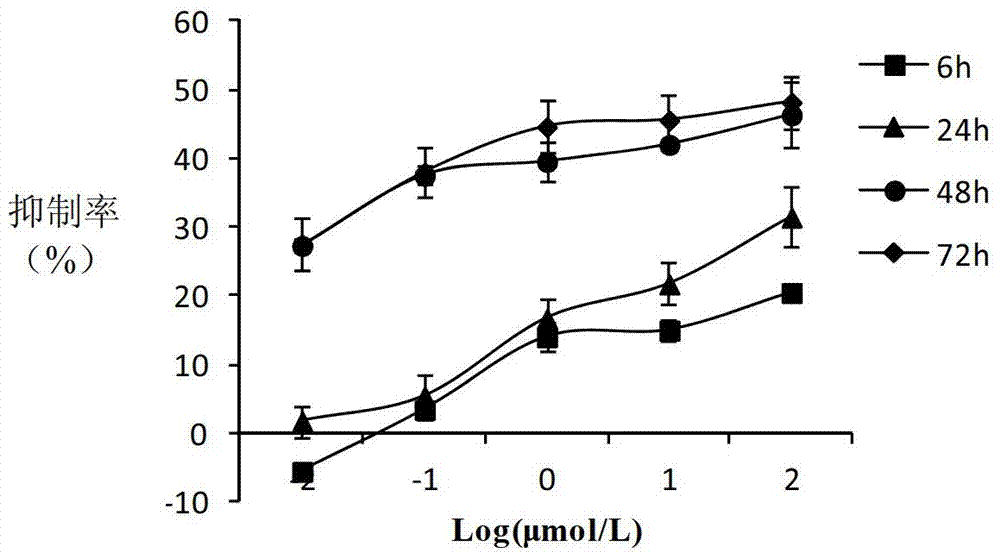
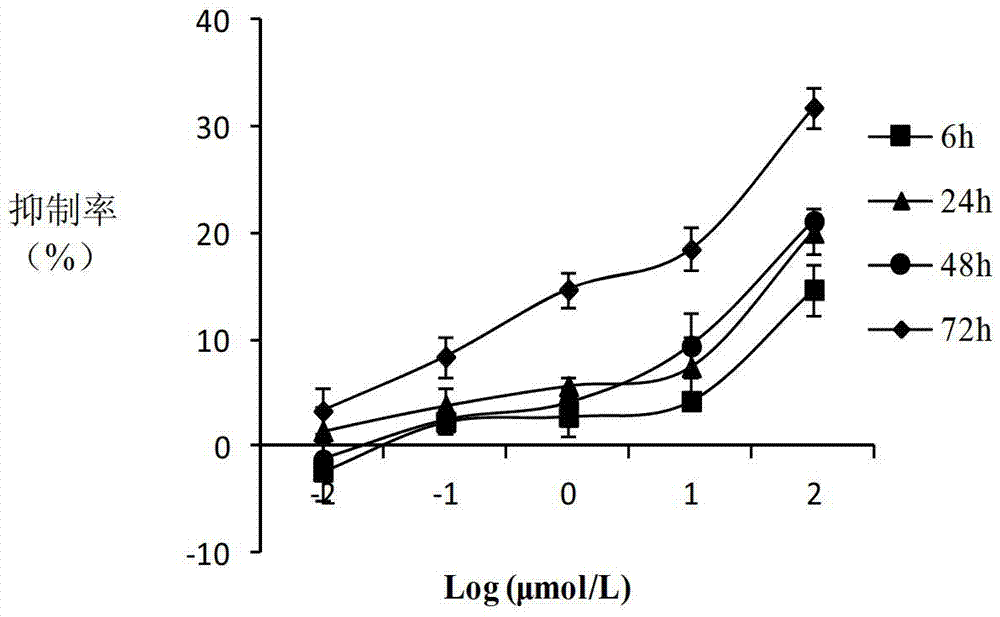
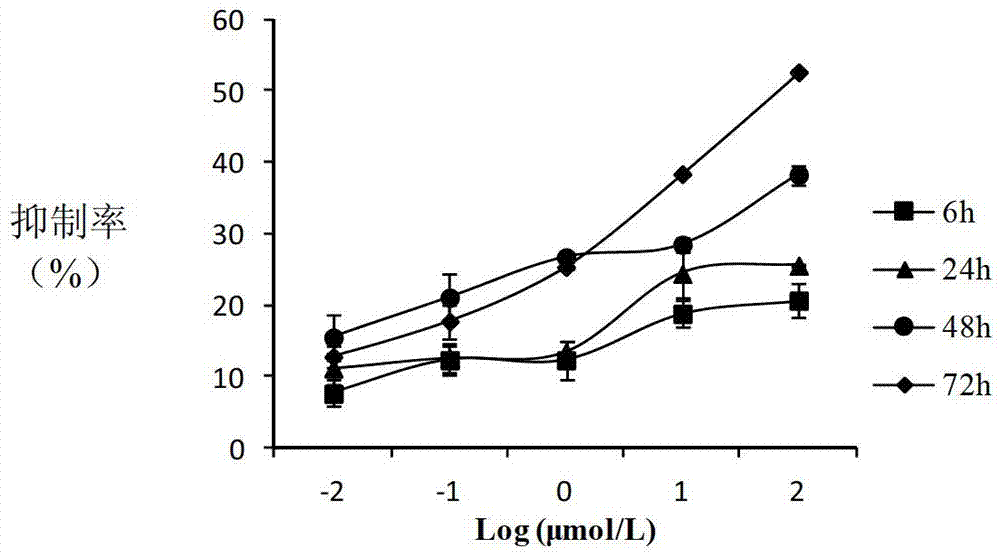
(20S)-camptothecin derivatives and application thereof
A technology of camptothecin and its derivatives, applied in the field of pesticides, can solve problems such as great influence on the natural control ability of natural enemies, threats to human health, and destruction of ecological balance, and achieve excellent contact activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Embodiment 1 camptothecin-20 (S)-O-2,2,3, the synthesis of 3-tetramethylcyclopropanyl carboxylate (a)
[0067] Describe the synthetic route of camptothecin-20(S)-O-2,2,3,3-tetramethylcyclopropanyl carboxylate (a) in detail below: add camptothecin (0.348g, 1mmol) and 4-dimethylaminopyridine (DMAP) (0.742g, 6mmol), pump out the air and replace it with N 2 Finally, 40 mL of dichloromethane and 2,2,3,3-tetramethylcyclopropanecarboxylic acid chloride (0.963 g, 6 mmol) were added, and the mixture was refluxed for 20 h under nitrogen protection. Add 80 mL of dichloromethane after the reaction, and then wash with 0.1 mol / L HCl. The organic phase was dried with anhydrous sodium sulfate, filtered and concentrated, and the solid was separated and purified by column chromatography (dichloromethane / methanol / petroleum ether=80 / 1 / 20), and finally 0.21 g of a light yellow solid was obtained. Yield 44.5%. 1 H NMR (DMSO-d 6 ,300MHz)δ0.94(6H,t,J=7.4Hz,H-18,CH 3 ),1.14(3H,s,CH 3 ),1....
Embodiment 2
[0069] Example 2 Synthesis of camptothecin-20 (S)-O-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanyl carboxylate (b)
[0070] The synthetic route of camptothecin-20(S)-O-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanyl carboxylate (b) is similar to a, and the column chromatography develops The agent is dichloromethane / methanol / petroleum ether=80 / 0.5 / 20), and finally 0.25 g of a light yellow solid was obtained, with a yield of 46.4%. 1 H NMR (DMSO-d 6 ,300MHz)δ0.96(3H,t,J=7.1Hz,H-18),1.12(3H,s,CH 3 ),1.20(3H,s,CH 3 ),1.31(1H,s,CHCOO),2.12(1H,s,CHCHCCl 2 ),2.21(2H,m,H-19),5.29(2H,s,H-5),5.49(2H,s,H-17),6.13(1H,m,CHCCl 2 ),6.99(1H,s,H-14),7.74(1H,t,J=7.0Hz,H-10),7.87(1H,t,H-11),8.13(each1H,t,J=7.5Hz ,H-12,H-7),8.67(1H,s,H-9); 13 C NMR (DMSO-d 6 ,75MHz)δ7.8(CH 3 ,C-18),20.1(CH 3 ,CH 3 ),20.2(CH 3 ,CH 3 ),21.9(C,C(CH 3 ) 2 ),22.0(CH,CHCHCCl 2 ), 30.2 (CH 2 ,C-19),33.0(C,CHCOO),50.5(CH 2 ,C-5),56.4(CH 2 ,C-17),76.3(C,C-20),94.8(CH,C-14),118.9(C,C-16),119.9(C,CHCCl 2...
Embodiment 3
[0072] Example 3 Camptothecin-20 (S)-O-3-(2-chloro-2-trifluoromethylvinyl)-2,2-dimethylcyclopropanyl carboxylate (c) synthesis
[0073] The synthetic route of camptothecin-20(S)-O-3-(2-chloro-2-trifluoromethylvinyl)-2,2-dimethylcyclopropanyl carboxylate (c) is similar to a, The developing solvent for column chromatography was ethyl acetate / petroleum ether=3 / 2, and finally 0.333 g of light yellow solid was obtained, with a yield of 58.1%. 1 H NMR (DMSO-d 6 ,300MHz)δ0.94(3H,t,J=7.4Hz,H-18),1.20(3H,s,CH 3 ),1.34(3H,s,CH 3 ),2.18(2H,m,H-19),2.37(1H,d,J=8.5Hz,CHCOO),2.56(1H,d,J=8.4Hz,CHCHC(CF 3 )Cl),5.28(2H,s,H-5),5.49(2H,s,H-17),6.59(1H,d,J=9.2Hz,CHC(CF 3)Cl),6.98(1H,s,H-14),7.73(1H,dd,J=8.0,1.0Hz,H-10),7.86(1H,t,J=1.4Hz,H-11),8.13 (each1H,t,J=8.1Hz,H-7,H-12),8.67(1H,s,H-9); 13 C NMR (CDCl 3 ,75MHz)δ7.2(CH 3 ,C-18),14.5(CH 3 ,CH 3 ), 18.8 (CH 3 ,CH 3 ),28.0(C,C(CH 3 ) 2 ),31.2(CH,CHCHC(CF 3 ) Cl), 31.6 (CH 2 ,C-19),32.0(CH,CHCOO),49.6(CH 2 ,C-5),66.8(CH 2 ,C-17),...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com