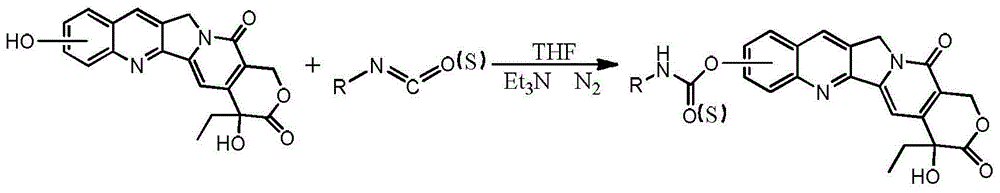Camptothecin derivative and preparation method thereof
A derivative, camptothecin technology, applied in the field of pesticides, can solve the problems of great influence on the natural control ability of natural enemies, environmental pollution, unreasonable use of chemical pesticides, etc., and achieve the effect of excellent contact activity and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
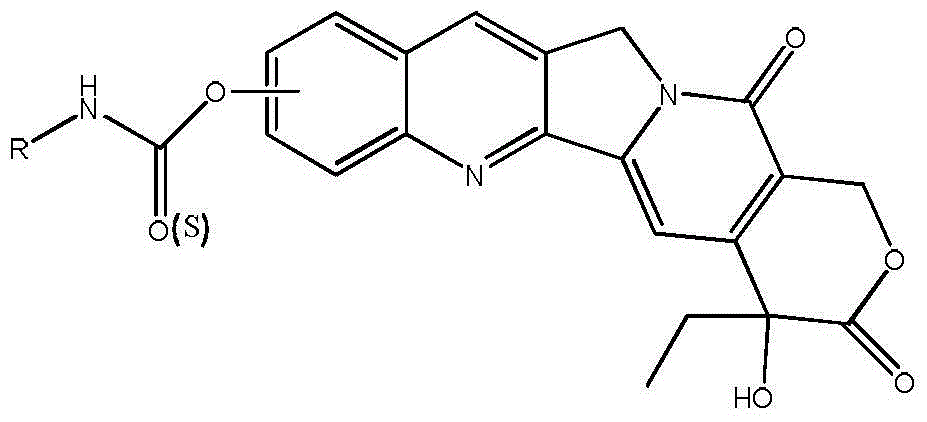
[0034] The present invention also provides a preparation method of the camptothecin derivative, and its synthetic route is as follows:
[0035]
[0036] Wherein, R is selected from one of C1-C8 linear alkanes and monohalogenated C1-C8 linear alkanes.
Embodiment 1
[0037] Example 1 (Preparation method of compound 27)
[0038] synthetic route
[0039]
[0040] Dissolve 364mg (1mmol) of 10-hydroxycamptothecin in 120ml of anhydrous tetrahydrofuran (THF), add 0.5ml of triethylamine, take out the air and change into N 2 Then, 430 mg (6 mmol) of propyl isocyanate was slowly added dropwise, and the reaction was carried out at room temperature for 24 hours. After the reaction is over, add 80mL of dichloromethane, dry the organic phase with anhydrous sodium sulfate, then filter, rotary evaporation and concentrate the organic layer to obtain a solid. Finally, the concentrated solid is separated and purified by silica gel column chromatography (the elution machine is two Methyl chloride / methanol / petroleum ether=80 / 1 / 20) to obtain 0.17 g of a light yellow solid.
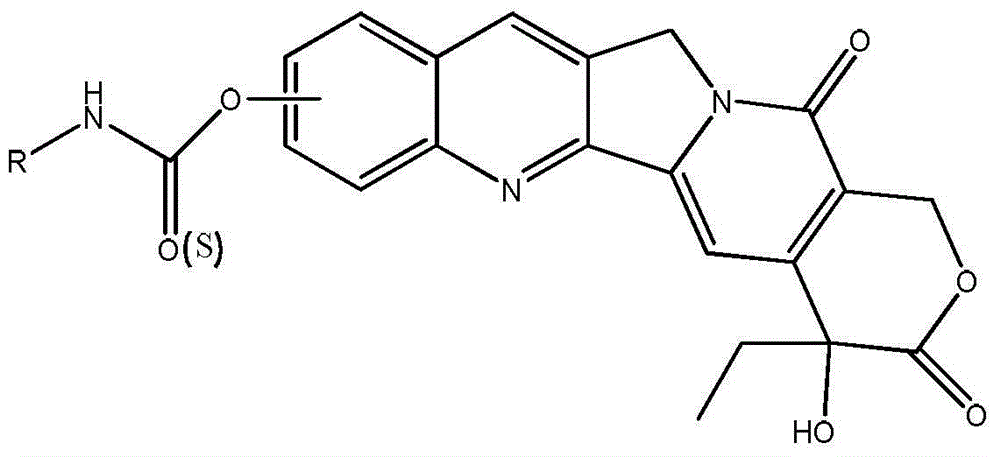
[0041] The structural formula of the resulting product is:
[0042]
[0043] NMR data: 1 H NMR (DMSO-d6, 300MHz) δ 0.89 (6H, m, H-18), 1.53 (2H, m), 1.87 (2H, m, H-19), 3.09 (2H, m), 5.30 (2H , ...
Embodiment 2
[0044] Example 2 (Preparation method of compound 134)
[0045] synthetic route
[0046]
[0047] Dissolve 364mg (1mmol) of 10-hydroxycamptothecin in 120ml of anhydrous tetrahydrofuran (THF), add 0.5ml of triethylamine, take out the air and change into N 2 Then, 723 mg (6 mmol) of ethyl chloroisocyanate was slowly added dropwise, and the reaction was carried out at room temperature for 24 hours. After the reaction is over, add 80mL of dichloromethane, dry the organic phase with anhydrous sodium sulfate, then filter, rotary evaporation and concentrate the organic layer to obtain a solid. Finally, the concentrated solid is separated and purified by silica gel column chromatography (the elution machine is two Methyl chloride / methanol / petroleum ether=80 / 1 / 20) to obtain 0.22 g of light yellow solid.
[0048] The structural formula of the resulting product is:
[0049]
[0050] NMR data: 1 H NMR (DMSO-d6, 300MHz) δ 0.87 (3H, m, H-18), 1.88 (2H, m, H-19), 3.58 (2H, m), 3.71 (2H, m), 5.29 (2H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com