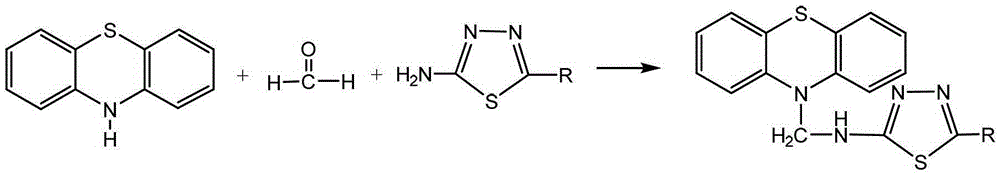
Compounds containing phenothiazinyl and 1,3,4-thiadiazolyl mannich bases and their preparation methods and applications
A phenothiazine-based and thiadiazolyl-based technology, applied in the field of chemical synthesis, can solve the problems of short reaction time, long reaction time, and low yield, and achieve the effects of short reaction time, simple post-treatment, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 1) Add 0.001 mol of phenothiazine, 0.002 mol of formaldehyde solution with a mass concentration of 37% to 40% (manufacturer: Tianjin Best Chemical Co., Ltd.) and 0.001 mol of 2-amino-1 into a dry three-necked flask, 3,4-thiadiazole, then add 10 mL of ethanol with a mass concentration of 95%, and stir and react at room temperature for 5 hours. At this time, when TLC monitors that the raw material point of phenothiazine disappears, the reaction solution is obtained; The developer is a mixture of ethyl acetate and petroleum ether, and the volume ratio of ethyl acetate and petroleum ether is 1:5;
[0038] 2) Evaporate the solvent in the reaction solution, and the residue in the three-necked flask is the crude product, which is recrystallized with absolute ethanol to obtain 2-(N-methylphenothiazine)amino-1 in the form of white powder, 3,4-Thiadiazole, 76% yield.
[0039] IR (KBr pellet method, ν / cm -1 ): 3487, 3088, 1648, 1601, 1447, 1327, 763.
[0040] 1 HNMR (CDCl 3 ,...
Embodiment 2
[0042]1) Add 0.001 mol of phenothiazine, 0.003 mol of formaldehyde solution with a mass concentration of 37% to 40% (manufacturer: Tianjin Best Chemical Co., Ltd.) and 0.001 mol of 2-amino-5- Methyl-1,3,4-thiadiazole, then add 10 mL of ethanol with a mass concentration of 95%, and stir and react at room temperature for 6 hours. At this time, when TLC monitors that the raw material point of phenothiazine disappears, the reaction solution is obtained ; Wherein, the developer of TLC is mixed by ethyl acetate and sherwood oil, and the volume ratio of ethyl acetate and sherwood oil is 1:5;
[0043] 2) Evaporate the solvent in the reaction solution, and the residue in the three-necked flask is the crude product, which is recrystallized with absolute ethanol to obtain 2-methyl-5-(N-methylphenothiazine in the form of white powder ) amino-1,3,4-thiadiazole with a yield of 90%.
[0044] IR (KBr pellet method, ν / cm -1 ): 3487, 3085, 2972, 2871, 1650, 1602, 1449, 1333, 768.
[0045] 1...
Embodiment 3
[0047] 1) Add 0.001 mol of phenothiazine, 0.003 mol of formaldehyde solution with a mass concentration of 37% to 40% (manufacturer: Tianjin Best Chemical Co., Ltd.) and 0.002 mol of 2-amino-5- Ethyl-1,3,4-thiadiazole, and then add 11 mL of ethanol with a mass fraction of 95%, and stir and react at room temperature for 6 hours. At this time, when TLC monitors that the raw material point of phenothiazine disappears, the reaction solution ; Wherein, the developer of TLC is mixed by ethyl acetate and sherwood oil, and the volume ratio of ethyl acetate and sherwood oil is 1:5;
[0048] 2) Evaporate the solvent in the reaction solution, and the residue in the three-necked flask is the crude product, which is recrystallized with absolute ethanol to obtain 2-ethyl-5-(N-methylphenothiazine ) amino-1,3,4-thiadiazole with a yield of 80%.
[0049] IR (KBr pellet method, ν / cm -1 ): 3489, 3090, 2970, 2928, 2873, 2855, 1650, 1603, 1449, 1330, 766.
[0050] 1 HNMR (CDCl 3 , 400M, TMS int...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com