Patch
A technology of patches and liquid additives, which can be used in medical preparations of non-active ingredients, cardiovascular system diseases, organic active ingredients, etc., and can solve problems such as inapplicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
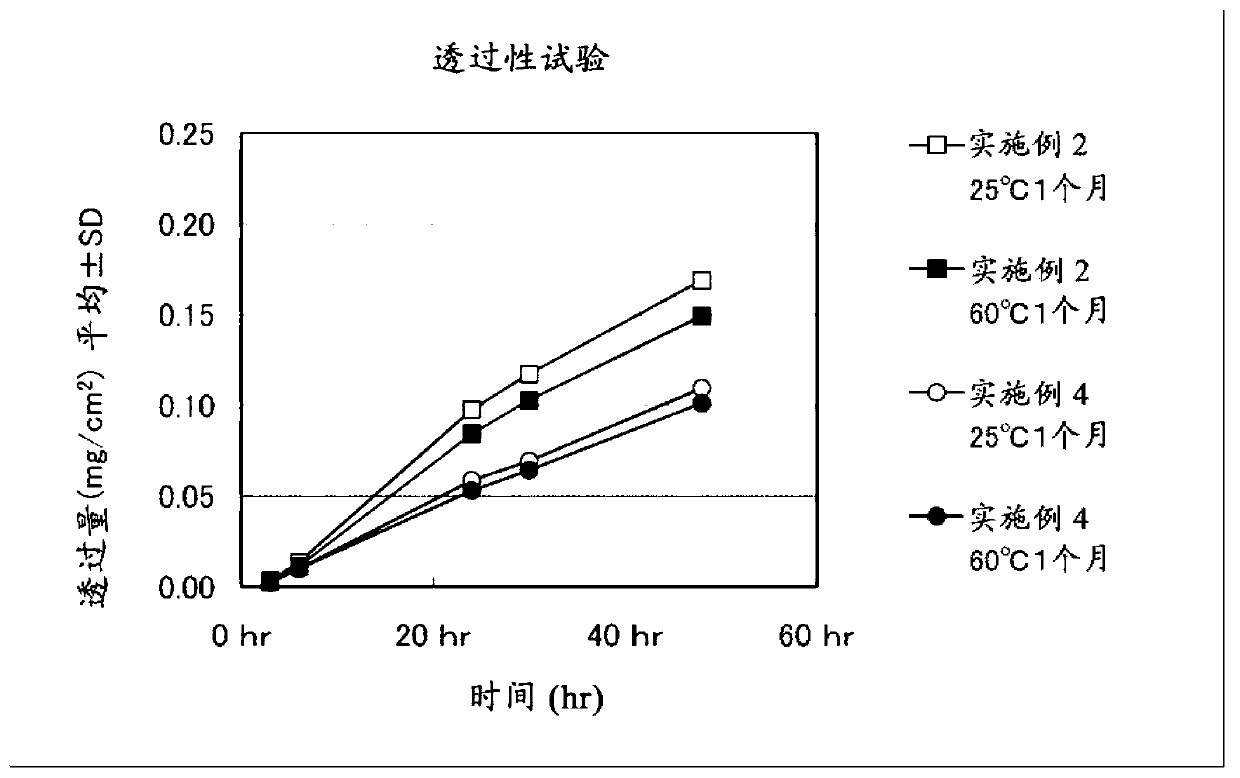
Embodiment 1
[0098] Get liquid additive (liquid paraffin, manufactured by Kaneda Company, trade name Hicall M72) 39 parts by weight, clonidine 13 parts by weight, light silicic anhydride (manufactured by Japan Aerosil Company, trade name Aerosil200) 8 parts by weight and toluene 560 parts by weight, Stir until homogeneous to obtain a mixture. Then, add 28 parts by weight of a polymer base (A) with a viscosity average molecular weight of more than 800,000 (high molecular weight polyisobutylene: viscosity average molecular weight 1,100,000, manufactured by BASF Company, trade name Oppanol B100) and a viscosity average molecular weight of 1 to the above mixed solution. More than 10,000 and less than 800,000 polymer base (B) (low molecular weight polyisobutylene: viscosity average molecular weight 36,000, manufactured by BASF, trade name Oppanol B10SFN) 12 parts by weight, mixed uniformly, and prepared a solution for forming a paste layer. The obtained paste layer-forming solution was coated o...
Embodiment 2
[0100] Get liquid additive (liquid paraffin, manufactured by Kaneda Company, trade name Hicall M72) 38 weight parts, clonidine 3 weight parts, light silicic anhydride (Japan Aerosil company manufactures, trade name Aerosil200) 9 weight parts and toluene 560 weight parts, Stir until homogeneous to obtain a mixture. Then, add 35 parts by weight of a polymer base (A) with a viscosity average molecular weight of more than 800,000 (high molecular weight polyisobutylene: viscosity average molecular weight 1,100,000, manufactured by BASF Company, trade name Oppanol B100) and a viscosity average molecular weight of 1 to the above mixed solution. More than 10,000 and less than 800,000 polymer base (B) (low molecular weight polyisobutylene: viscosity average molecular weight 36,000, manufactured by BASF, trade name Oppanol B10SFN) 15 parts by weight, mixed uniformly, and prepared a solution for forming an adhesive layer. The obtained solution for forming an adhesive layer was coated on ...
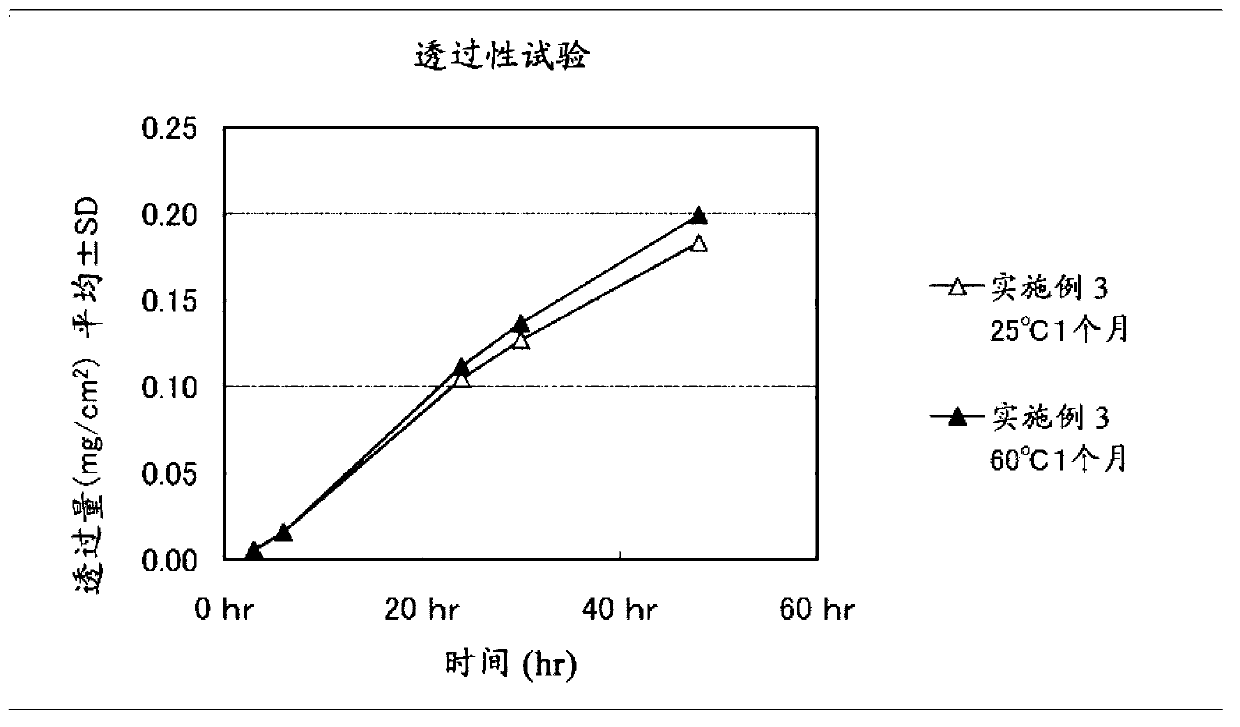
Embodiment 3
[0102] Get 47 parts by weight of liquid additive (liquid paraffin, manufactured by Kaneda Company, trade name Hicall M72), 13 parts by weight of clonidine, 8 parts by weight of light silicic anhydride (manufactured by Aerosil Corporation of Japan, trade name Aerosil200), and 560 parts by weight of toluene, Stir until homogeneous to obtain a mixture. Then, add 29 parts by weight of a polymer base (A) with a viscosity average molecular weight of more than 800,000 (high molecular weight polyisobutylene: viscosity average molecular weight 1,100,000, manufactured by BASF Company, trade name Oppanol B100,) in the above-mentioned mixed solution. More than 10,000 and less than 800,000 polymer base (B) (low molecular weight polyisobutylene: viscosity average molecular weight 36,000, manufactured by BASF Corporation, trade name Oppanol B10SFN) 3 parts by weight, mixed uniformly, and prepared a solution for forming a paste layer . The obtained paste layer-forming solution was coated on ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com