Application of sterol phenolic ester to preparation of breast cancer prevention and treatment medicine
A technology of sterol phenolic acid ester and dihydrositosterol ferulic acid ester, which is applied in the application field of sterol phenolic acid ester in the preparation of drugs for preventing and treating breast cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation method of sterol phenolic acid ester in embodiment 1, rice bran
[0025] Take 1000g of rice bran and extract it three times with acetone (each time: the amount of acetone used is 10L, the extraction temperature is 50°C, and the extraction time is 4 hours), and the extracts obtained by combining the three times are spin-dried with a rotary evaporator (at 45°C), Take the spin-dried product and dissolve it in methanol solution of KOH (1000ml, the concentration of KOH is 2mol / L), and extract it with n-hexane at room temperature for 3 times (each time: the amount of n-hexane is 1000ml, and the extraction time is 10 minutes) , collect the methanol phase (located in the lower layer) obtained 3 times, add hydrochloric acid (concentration: 2mol / L, dosage: 1000ml) to acidify, then use n-hexane to extract 3 times at room temperature (each time: the dosage of n-hexane is 1000ml, and the extraction time is 10 Minutes), the obtained n-hexane phase (located in the uppe...
Embodiment 2
[0027] The preparation method of sterol phenolic acid ester in embodiment 2, corn bran
[0028] With reference to the method described in Example 1:
[0029] Take 2000g of corn bran, extract it three times with acetone, spin the extract to dryness with a rotary evaporator, dissolve the spin-dried residue in methanol solution of KOH, and extract three times with n-hexane, collect the methanol phase, add hydrochloric acid to acidify it, and use n-hexane The n-hexane phase was extracted three times, and the n-hexane phase was collected, blown to dry with nitrogen to obtain sterol phenolate, and the total amount was 64.1 g.
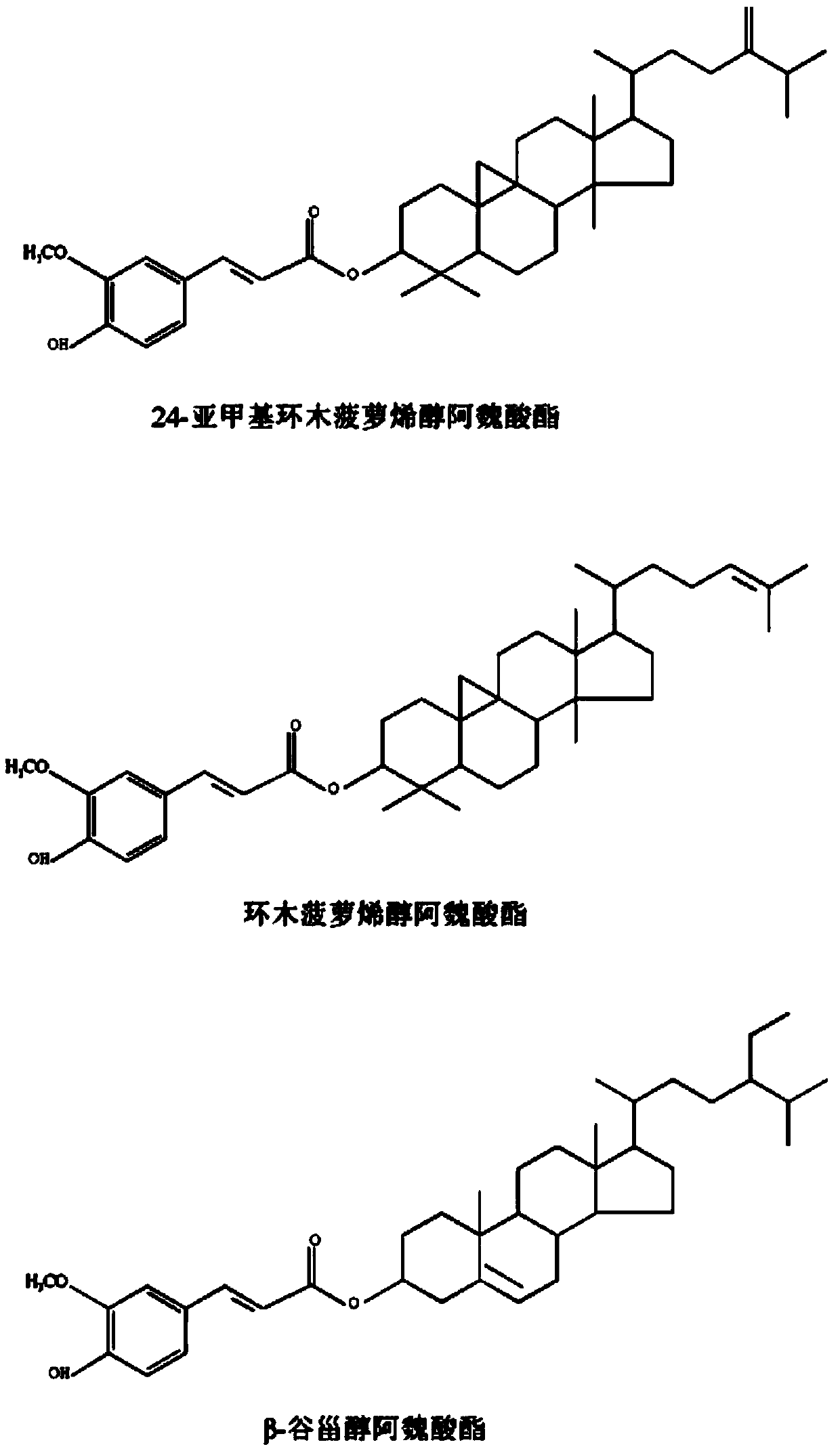
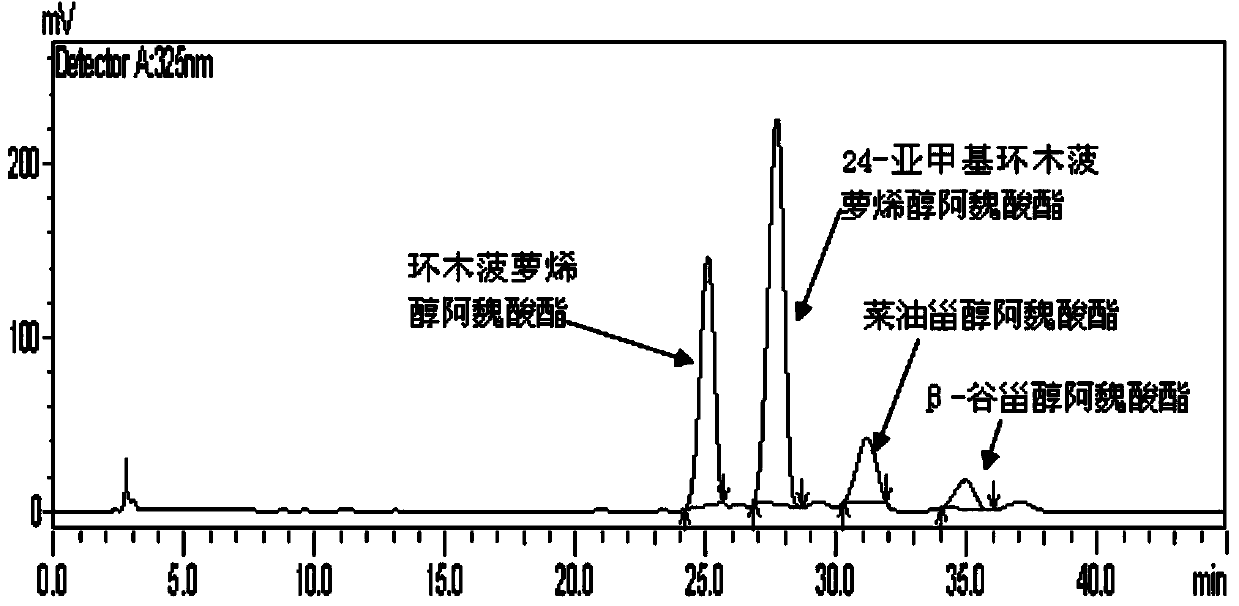
[0030] According to the quantitative analysis by HPLC, the sterol phenolic esters in corn hulls contain 24-methylenecycloartenol ferulate, cycloartenol ferulate, β-sitosterol ferulate , dihydrositosterol ferulate and campesterol ferulate, the contents of which are 5%, 4.5%, 20%, 10% and 15% respectively, which account for 54.5% of the total.
Embodiment 3
[0031] The preparation method of sterol phenoxylate in embodiment 3, bran
[0032] With reference to the method described in Example 1:
[0033] Take 3000g of bran, extract three times with acetone, spin dry the extract with a rotary evaporator, dissolve the spin-dried residue in methanol solution of KOH, and extract three times with n-hexane, collect the methanol phase, acidify with hydrochloric acid, and use n-hexane The n-hexane phase was extracted three times, and the n-hexane phase was collected, blown to dry with nitrogen to obtain sterol phenolic acid ester, and the total amount was 43.5 g.
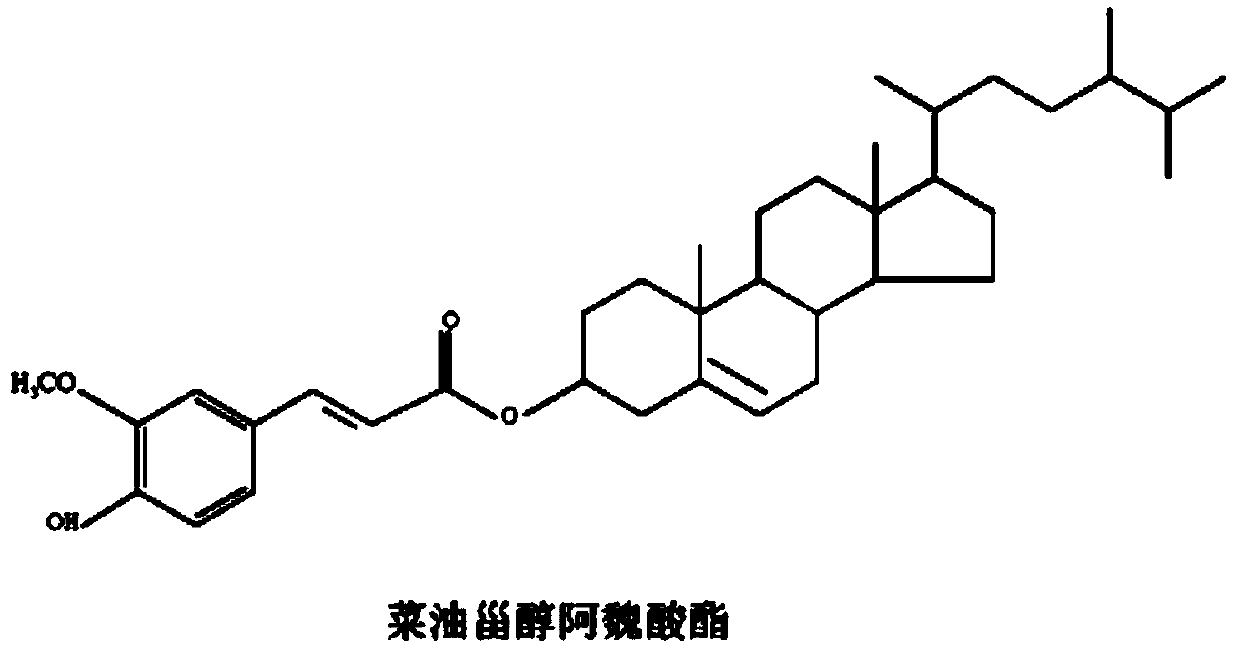
[0034]According to the quantitative analysis by HPLC, the sterol phenolic esters in the bran contain stigmasteryl ferulate, cycloartenol ferulate, β-sitosteryl ferulate (β-Sitosteryl ferulate) and rapeseed oil The content of sterol ferulic acid ester is 10%, 2%, 1%, 25% respectively, which accounts for 38% of the total.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com