Method for synthesizing isolongifolene by catalytically isomerizing longifolene with solid acid
A solid acid catalyzed, isolongifolene technology, applied in the direction of isomerization hydrocarbon production, organic chemistry, chemical recovery, etc., can solve the problems of pollution, high production costs, etc., to reduce corrosion, save labor and provide competitiveness Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
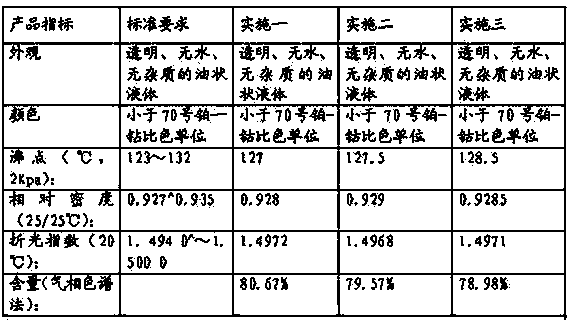
[0009] The method for the synthesis of isolongifolene by solid acid catalyzed isomerization of longifolene, the small test feed is 100 grams of longifolene / pot: 20 grams of macroporous strong acid cation exchange resin D72 catalyst is added to a 500ml three-necked flask, and then 100 grams of 65% longifolene and 80 grams of glacial acetic acid are mixed and added to the reaction flask, stirring is started to heat, and the isomerization reaction is carried out in the reaction kettle. For the content of leafene, take samples every 0.5-1 hour until the content of isolongifolene no longer increases or increases very slowly or even falls back, then stop adding and start cooling down to below 40 degrees. The reaction lasts for about 4 hours, and then sequentially Filtration was carried out, the catalyst was recovered, and finally the filtrate was subjected to atmospheric distillation to recover the solvent, and the analysis results of the crude isolongifolene obtained were: the conte...
Embodiment 2
[0012] The method for the synthesis of isolongifolene by solid acid catalyzed isomerization of longifolene, enlarged test 10000 grams of longifolene / pot: 3000 grams of macroporous strong acid cation exchange resin D72 catalyst is added in a 30L reactor, and then 10000 Gram 65% longifolene and 12000 grams of glacial acetic acid are added respectively to the reaction kettle, start stirring, heating, carry out isomerization reaction in the reaction kettle, the temperature in the reaction kettle is 90 ℃, after 3 hours, sampling analysis isolongifolene content , sampling once every 0.5-1 hour, until the content of isolongifolene no longer increases or increases very slowly or even falls back, stop adding and start to cool down, cool down to below 40 degrees, react for a total of about 5 hours, and then filter in turn, The catalyst was recovered, and finally the filtrate was subjected to atmospheric distillation to recover the solvent, and the analysis results of the crude isolongifo...
Embodiment 3
[0015] The method for synthesizing isolongifolene by solid acid catalyzed isomerization longifolene, pilot test 100kg longifolene / pot, 10kg of macroporous strong acid cation exchange resin D72 catalyst is added in the pilot test reactor, and then 100kg65% longifolene Folene and 60kg of glacial acetic acid are added to the reactor respectively, start stirring and heating, and carry out isomerization reaction in the reactor. Sampling once every 1 hour, until the isolongifolene content no longer increases or increases very slowly or even drops back down, stop adding and start to cool down, cool down to below 40 degrees, and react for about 6 hours in total, then filter in turn, recover the catalyst, and finally put The filtrate was subjected to atmospheric distillation to recover the solvent, and the analysis results of the crude isolongifolene obtained were: the content of isolongifolene was 59.838%, and the content of longifolene was 2.9%. The crude isolongifolene is distilled ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com