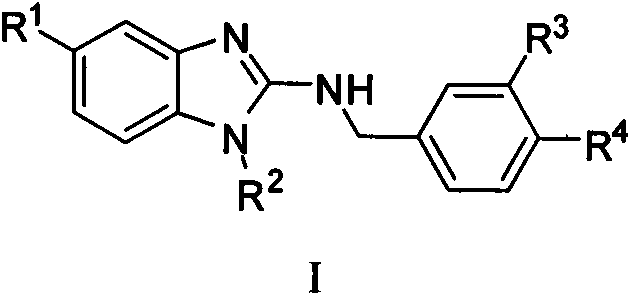
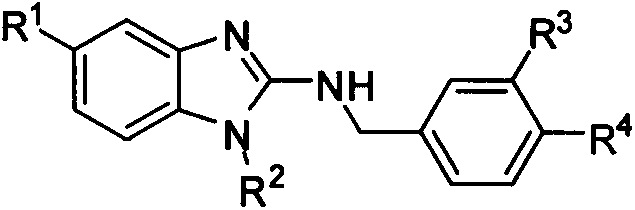
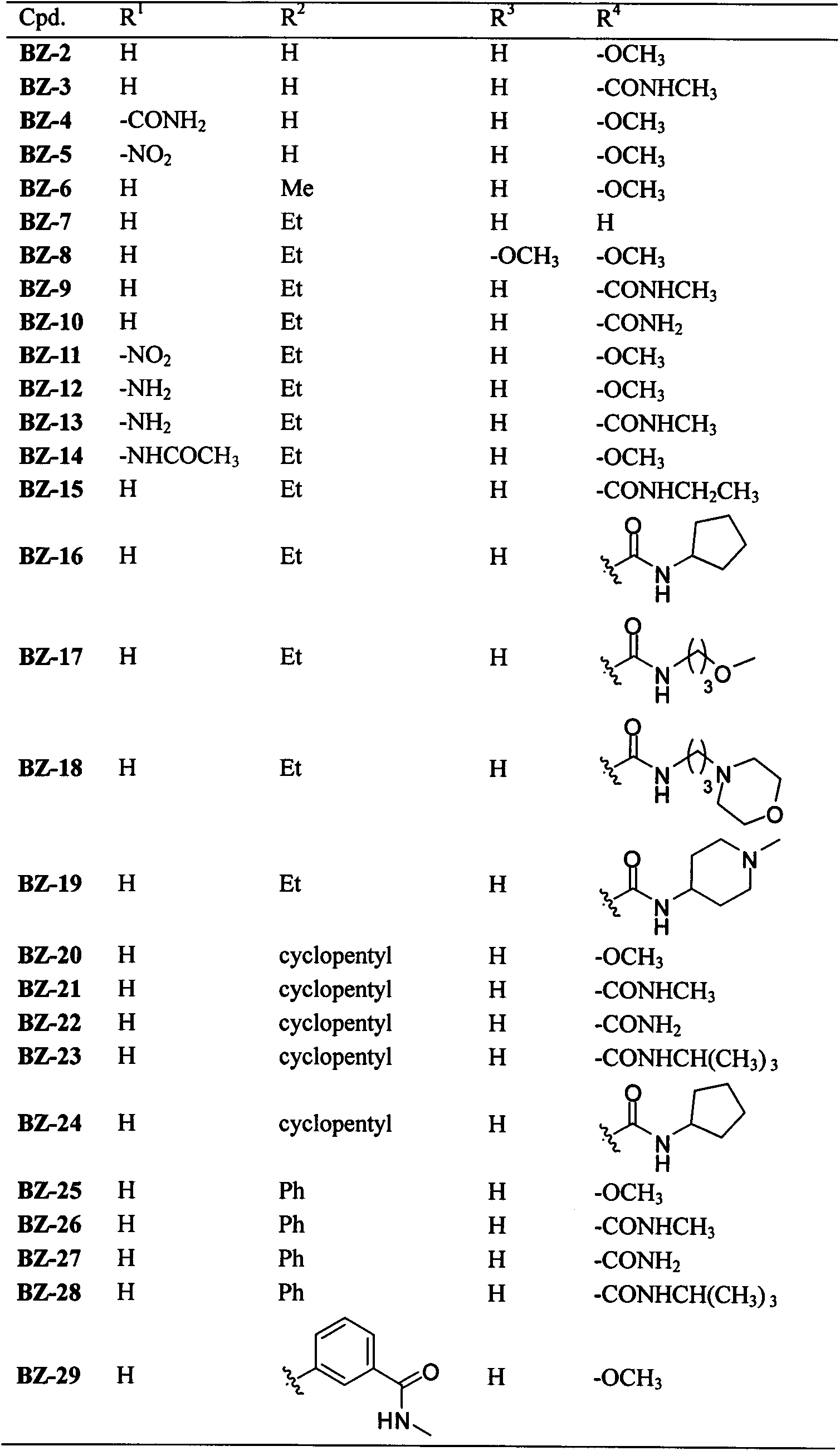
2-aminobenzimidazoles and applications thereof
A compound, benzo technology, applied in the field of structure with anti-tumor activity, can solve problems such as poor selectivity, toxicity and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1H-Benzo[d]imidazol-2-amine (1a)
[0041] Add 20ml of water, 5ml of acetonitrile and 0.70g (6.5mmol) of o-phenylenediamine into a 50ml reaction flask, stir evenly and slowly add 0.69g (6.5mmol) of BrCN in batches, heat up to 100°C after addition, reflux for 2h, TLC shows There is no remaining raw material, stop the reaction, adjust the pH to alkaline with ammonia water after cooling, precipitate white and deep, filter with suction, extract the filtrate with ethyl acetate (80ml×3), and use anhydrous MgSO for the organic phase 4 Dry, filter and concentrate under reduced pressure, combine with the filter cake, and recrystallize from ethanol to obtain 0.70 g of white crystals, yield 81.0%, mp.223~226°C, MS[M+H] + 134.05.
Embodiment 2
[0043] 5-Nitro-1H-benzo[d]imidazol-2-amine (1b)
[0044] The preparation method is similar to that of 1a, light red solid, yield 45.5%, mp.212~214°C.
Embodiment 3
[0046]2-Amino-1H-benzo[d]imidazole-5-carboxylic acid ethyl ester (1c)
[0047] The preparation method is similar to that of 1a, white solid, yield 62.9%, mp.216~218℃, MS[M+H] + 206.08.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com