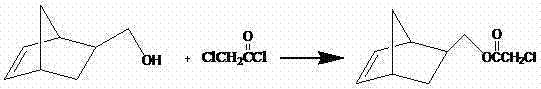Preparation method of 5-norborn-2-enyl methyl chloroacetate
A technology of norbornene methyl ester, applied in the field of preparation of norbornene methyl chloroacetate, can solve the problem of wasting raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] A preparation method of norbornene methyl chloroacetate, the steps are as follows:
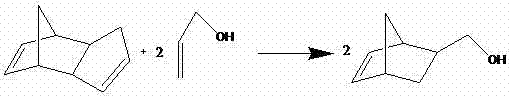
[0024] 1) Take 2.5mL (18.6mmol) of dicyclopentadiene and 3.0mL (44.1mmol) of allyl alcohol, add it to a 10mL tubular reactor, freeze it with liquid nitrogen until it is completely solidified, and then slowly melt it with nitrogen gas , repeat this cycle step 2 times to completely remove the air that may be dissolved in it, then freeze until solidified, vacuumize to 339Pa and seal the tube;
[0025] 2) Put the sealed tube into a constant temperature oil bath, slowly heat up to 150°C and react for 8 hours, then transfer the product in the tube into a round bottom flask, and distill under reduced pressure to 399Pa at room temperature to evaporate excess allyl in the system Alcohol, and then under reduced pressure, slowly warming up to 89 ° C, the collected fractions of colorless liquid is 5-hydroxymethyl-2-norbornene (NBCH 2 OH);
[0026] 3) Add 10mL of pure α-chloroacetyl chloride, 15mL...
Embodiment 2
[0030] A preparation method of norbornene methyl chloroacetate, the steps are as follows:
[0031] 1) Take 2.5mL (18.6mmol) of dicyclopentadiene and 3.0mL (44.1mmol) of allyl alcohol, add it to a 50mL reactor, freeze it with liquid nitrogen until it is completely solidified, then slowly melt it with nitrogen gas, repeat This cycle step is 3 times to completely remove the air that may be dissolved therein, then freeze until solidified, and then close the reactor after vacuuming to 339Pa;
[0032] 2) Put the reactor into a constant temperature oil bath, slowly heat up to 190°C and react for 10 hours, then transfer the product in the reactor into a round bottom flask, reduce the pressure to 399Pa at room temperature and distill off the excess olefin in the system Propanol, under reduced pressure, then slowly warming up to 89 ° C, the collected fractions are colorless liquids that are 5-hydroxymethyl-2-norbornene (NBCH 2 OH);
[0033] 3) Add 10mL of pure α-chloroacetyl chloride,...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap