Near-infrared light absorption polysulfide metal complexes and their preparation methods and applications
A technology of metal complexes and near-infrared light, which is applied in the field of near-infrared light-absorbing materials, can solve the problems of no absorption or weak absorption in the visible spectral region, difficult adjustment of the spectrum, cumbersome preparation, etc., and achieve easy mass production and good Effects of photochemical stability and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
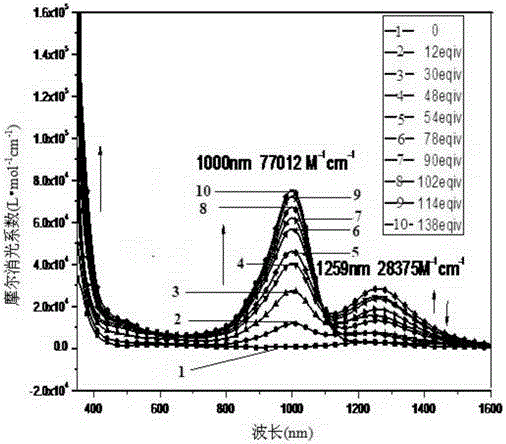
[0137] Compound III, when R' and R'' are both -C 8 h 17 , to prepare 4,8-dioctyl-1,3,5,7-tetrathiobenzobisindene-2,6-dione, denoted as compound III-1:
[0138] First add 4.61g (192mmol) of sodium metal strips and 12.34g (96mmol) of naphthalene to 200ml of anhydrous tetrahydrofuran, then add 9.52g (16mmol) of 3,6-dioctyl-1,2,4, 5-Tetraisopropylthiobenzene, protected by argon, stirred at 60°C for 16 hours. After the system was cooled to room temperature, 15ml of triethylamine was injected into the system in an ice-salt bath, and then 120ml containing 9.50g ( Add the toluene solution of 32mmol) triphosgene dropwise into the system, and the addition takes 2h. After the addition is completed, continue to stir for 1h, then naturally rise to room temperature, and slowly add 50ml of deionized water dropwise through the constant pressure dropping funnel, which takes 1h , then add 200ml of deionized water to wash the reaction solution, separate the liquid, extract the aqueous phase wi...
Embodiment 2
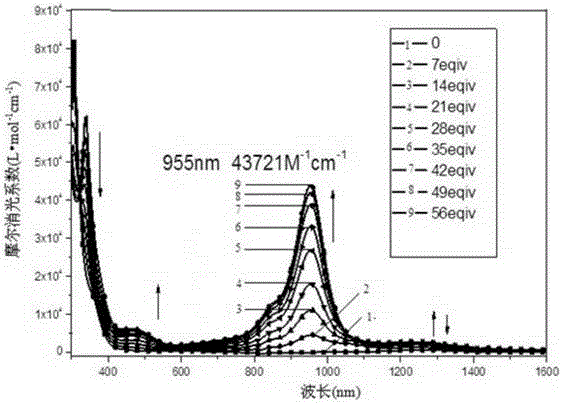
[0141] Compound III, when R' and R'' are both -OC 8 h 17 , to prepare 4,8-dioctyloxy-1,3,5,7-tetrathiobenzobisindene-2,6-dione, denoted as compound III-2:
[0142] First add 5.52g (240mmol) of sodium metal strips and 15.38g (120mmol) of naphthalene to 150ml of anhydrous tetrahydrofuran, then add 12.73g (20mmol) of 3,6-dioctyloxy-1,2,4 , 5-Tetraisopropylthiobenzene, protected by argon, stirred at 50°C for 24 hours, after the system was cooled to room temperature, 20ml of triethylamine was injected into the system in an ice-salt bath, and then 120ml containing 11.87g (40mmol) the toluene solution of triphosgene was added dropwise in the system, and it took 2.5h to add. After the addition was completed, continue to stir for 1h, then naturally rose to room temperature, and slowly added 50ml of deionized water through the constant pressure dropping funnel, After 1 hour, add 250ml of deionized water to wash the reaction solution, separate the liquids, extract the aqueous phase wit...
Embodiment 3
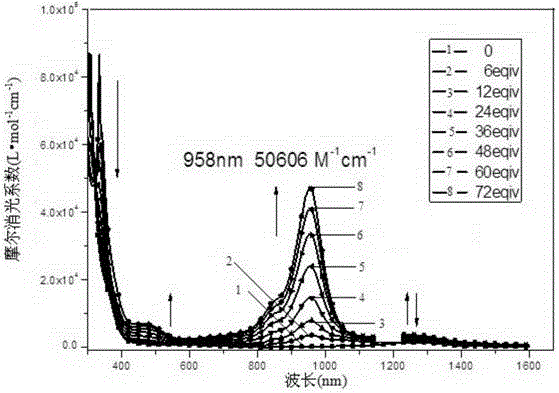
[0148] Compound III, when R' and R" are both -OC 6 h 13 , to prepare 4,8-dihexyloxy-1,3,5,7-tetrathiobenzobisindene-2,6-dione, denoted as compound III-3:
[0149] First add 2.3g (96mmol) of sodium metal strips and 6.17g (48mmol) of naphthalene to 120ml of anhydrous tetrahydrofuran, then add 4.59g (8mmol) of 3,6-dihexyloxy-1,2,4 , 5-Tetraisopropylthiobenzene, protected by argon, stirred at 45°C for 22 hours, after the system was cooled to room temperature, 7.5ml of triethylamine was injected into the system in an ice-salt bath, and then 70ml containing 4.75 The toluene solution of g (16mmol) triphosgene was added dropwise to the system, and the addition took 1.5h. After the addition was completed, continue to stir for 1h, then naturally rose to room temperature, and slowly added 50ml of deionized water through the constant pressure dropping funnel. , after 1 hour, add 120ml of deionized water to wash the reaction solution, separate the liquid, extract the water phase with dic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com