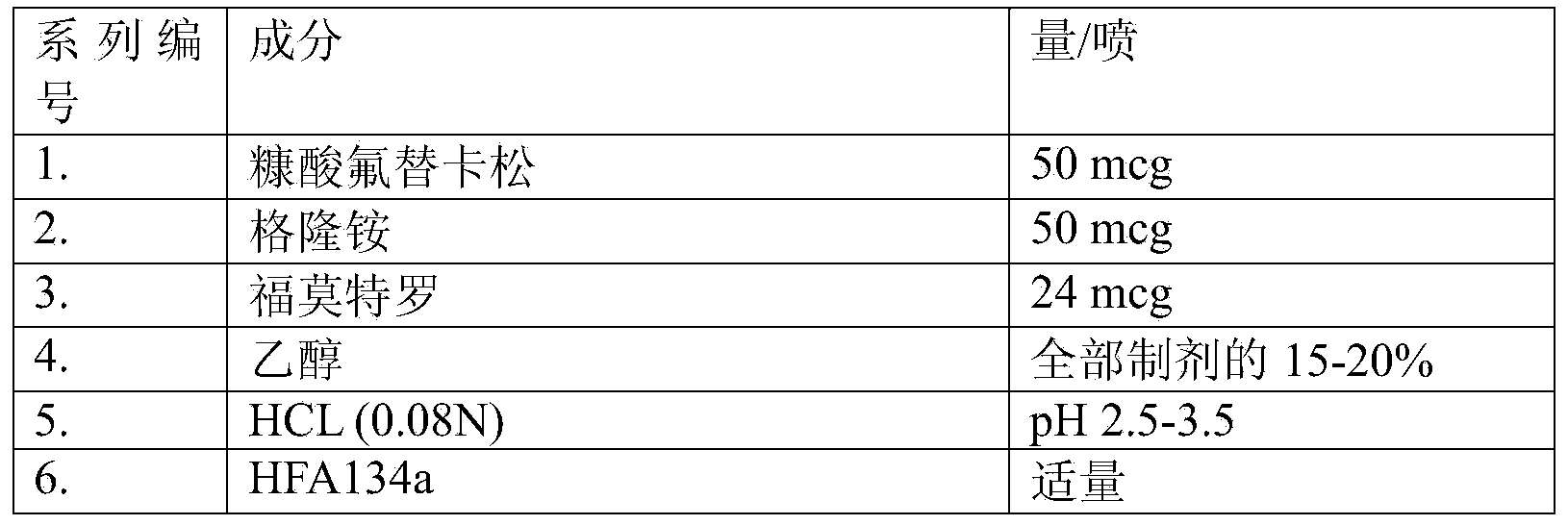
Composition of glycopyrrolate and a beta2-agonist
一种组合物、激动剂的技术,应用在炎性或阻塞性气道疾病,预防和/或治疗呼吸性,慢性阻塞性肺病领域,能够解决老年人骨密度不利影响、皮质类固醇使用限制等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0139] process:
[0140] 1) Homogenize fluticasone furoate, glycopyrronium and tiotropium bromide with a portion of HFA.
[0141] 2) Transfer the suspension obtained in step 1 to a mixing vessel and add the remaining amount of HFA.
[0142] 3) The resulting suspension was mixed, recycled and filled into pre-crimped aluminum cans.
Embodiment 2
[0145] process:
[0146] 1) Fluticasone furoate, indacaterol and glycopyrronium were homogenized with lactose and a portion of HFA.
[0147] 2) Transfer the suspension obtained in step 1 to a mixing vessel and add the remaining amount of HFA.
[0148] 3) The resulting suspension was mixed, recycled and filled into pre-crimped aluminum cans.
Embodiment 3
[0151] process:
[0152] 1) Dissolve PVP in PEG and a partial amount of HFA134A or HFA227.
[0153] 2) Transfer the solution obtained in step 1 to a mixing vessel.
[0154] 3) Fluticasone furoate, indacaterol and glycopyrronium were homogenized with a portion of HFA.
[0155] 4) Transfer the suspension obtained in step 3 to a mixing vessel and add the remainder of HFA.
[0156] 5) The entire resulting suspension was mixed, recycled and filled into pre-crimped aluminum cans.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com