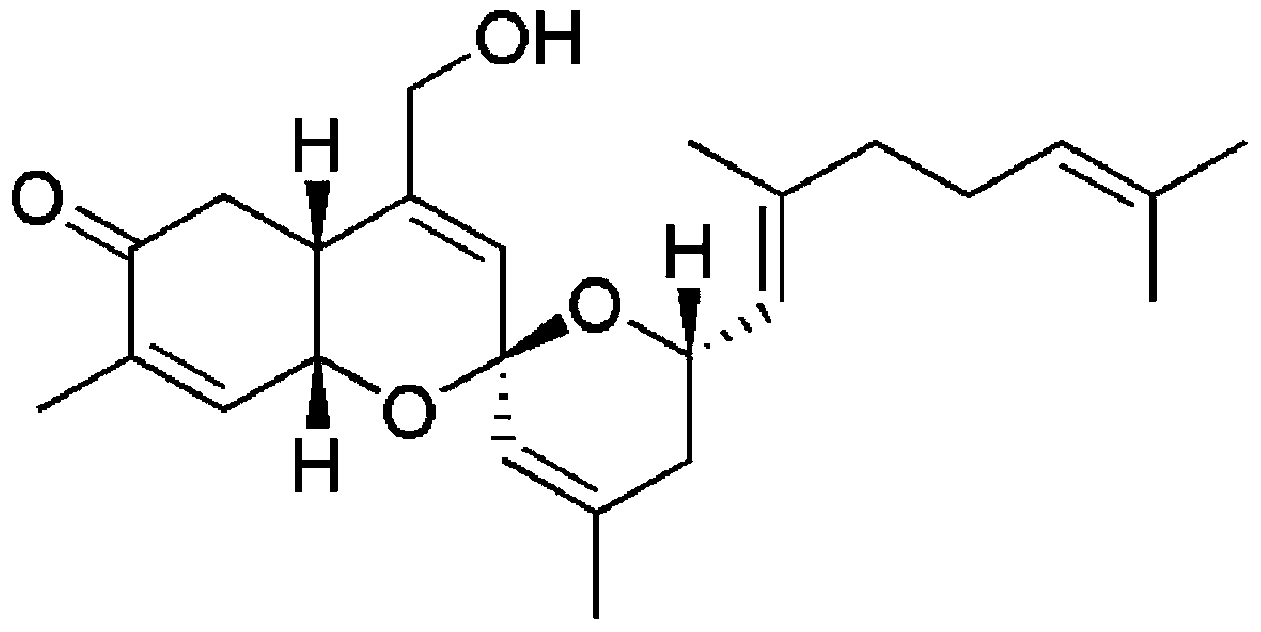
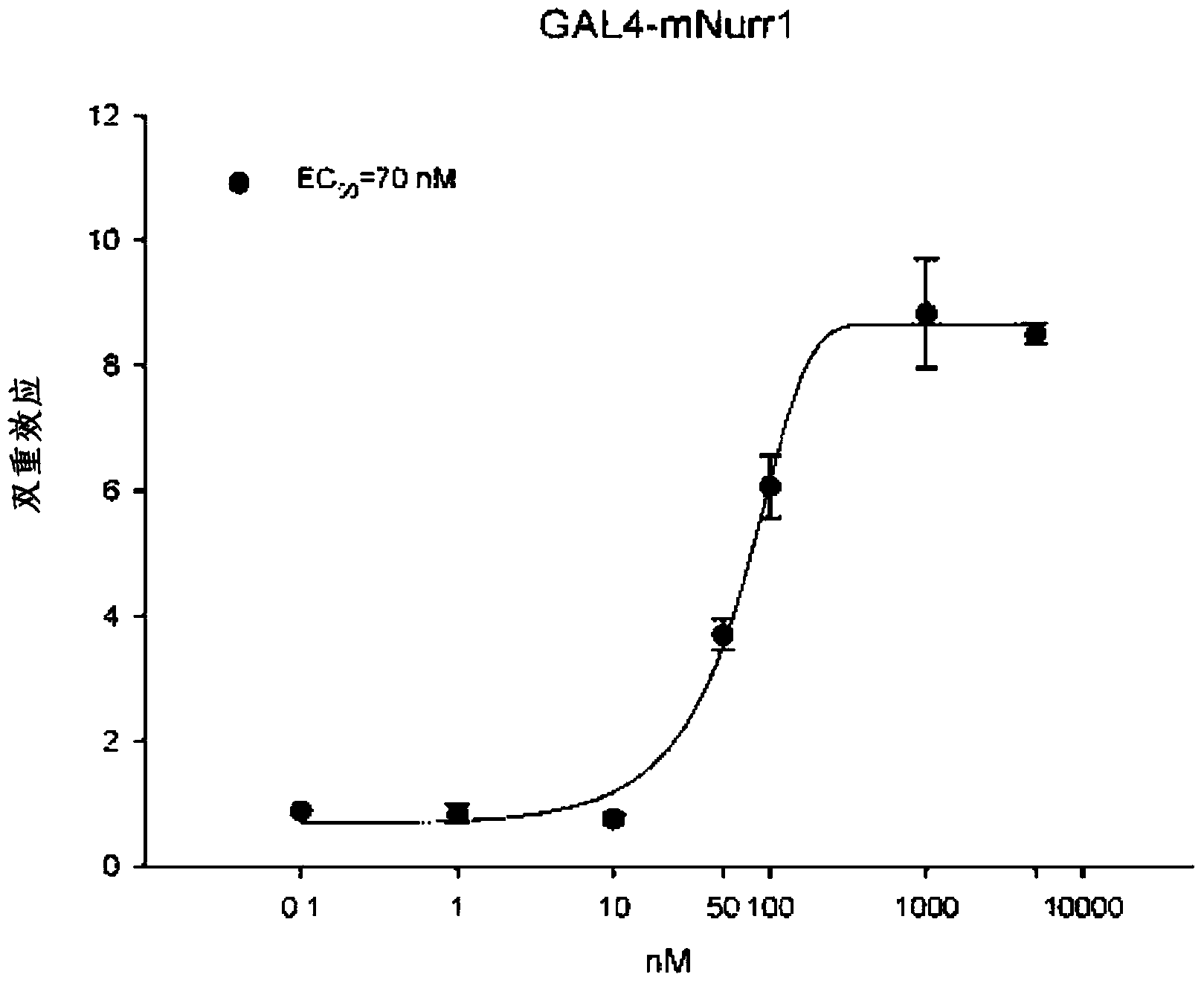
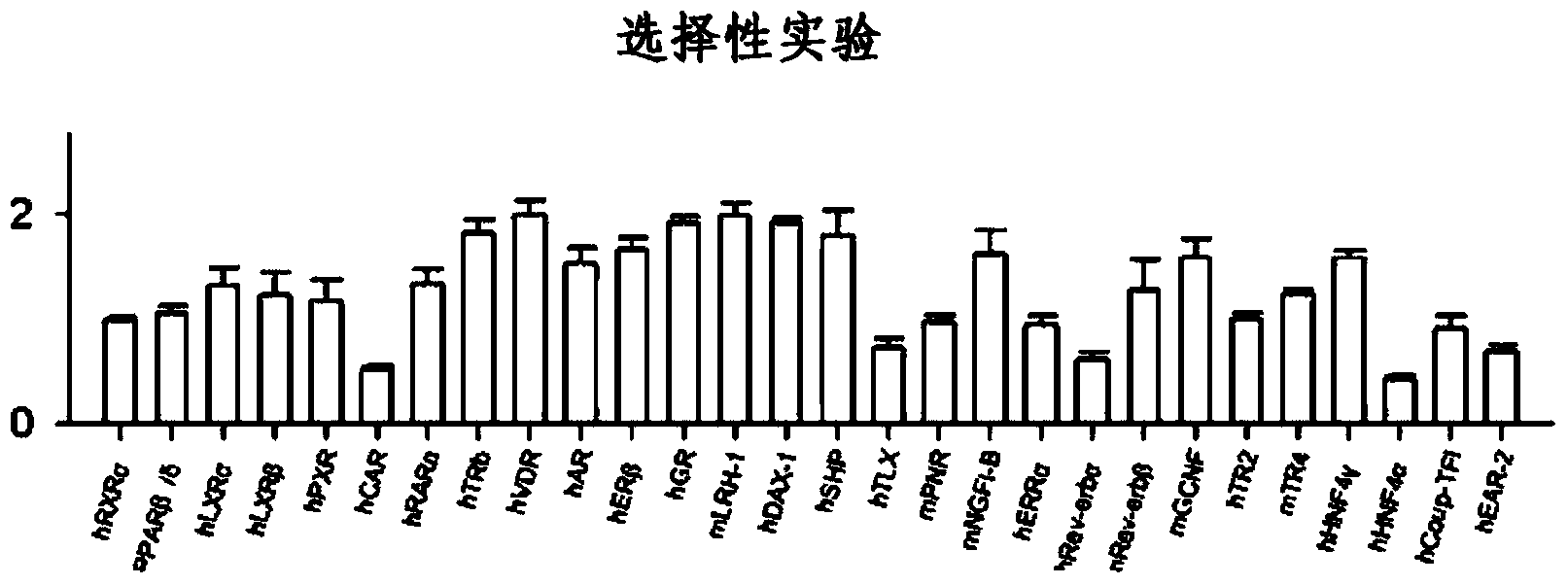
Sesterterpene compounds and use thereof
A technology for disesquiterpenes and compounds, applied in the field of novel disesquiterpenes, can solve problems such as incompatibility, and achieve the effects of inhibiting the production of fatty acids in the liver, inhibiting the differentiation of adipocytes, and reducing the accumulation of fat
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] Embodiment 1. Synthesis of compound 1
[0115]
[0116] PhorbaketalA (phorbaketalA, A) (10 mg, 0.025 mmol, Rho et.al., Organic Letters, 2009, 11, 5590-5593 (Rho et al., 2009, 11, 5590-5593)) was dissolved in dichloromethane, and added p-TsCL (5.4 mg, 1.2 eq) and triethylamine (0.01 mmol) were stirred for 5 hours. with saturated NaHCO 3 Aqueous solution and water (40ml) quenched the reaction. The organic solvent layer was washed twice with water, using Na 2 SO 4 After drying, it was concentrated with an evaporator. Compound 1 was obtained by purification with silica gel column chromatography. MS m / z554[M+H] +
Embodiment 2
[0117] Embodiment 2. Synthesis of compound 2
[0118]
[0119] Dissolve compound 1 (10 mg, 0.018 mmol) in dimethylformamide (DMF, dimethylformamide, 5 ml), add NaN 3 (11.7mg, 10mmol) and react at 70°C for 8 hours under nitrogen. After the reaction solution was cooled, ice water was added, and the organic solvent layer was separated after washing with water. will use Na 2 SO 4 The residue obtained by drying and distilling under reduced pressure was purified by silica gel column chromatography to obtain Compound 2. MS m / z424[M+H] +
Embodiment 3
[0120] Embodiment 3. Synthesis of compound 3
[0121]
[0122] Dissolve compound 2 (10mg, 0.023mmol) in acetonitrile (5ml) and add NaI (0.20mmol) and FeCl 3 (0.032 mmol). After stirring the reaction solution for 20 minutes, chloroform (5 ml) was added to terminate the reaction. Utilize Na 2 SO 3 Aqueous solution and NaHCO 3 Washed with aqueous solution, and the obtained organic solvent layer was washed with brine. The organic solvent layer was washed with Na 2 SO 4 The residue obtained by drying and distilling under reduced pressure was purified by silica gel column chromatography to obtain compound 3. MS m / z398[M+H] +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com