Method for separating endothelial colony-forming cells
A separation method and cell technology, applied in the direction of animal cells, vertebrate cells, artificial cell constructs, etc., can solve the problems of inability to separate cells and collagen tissues, insufficient separation by enzymatic digestion, and low separation efficiency, reaching clinical Excellent application prospects, high separation efficiency, and large number of cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
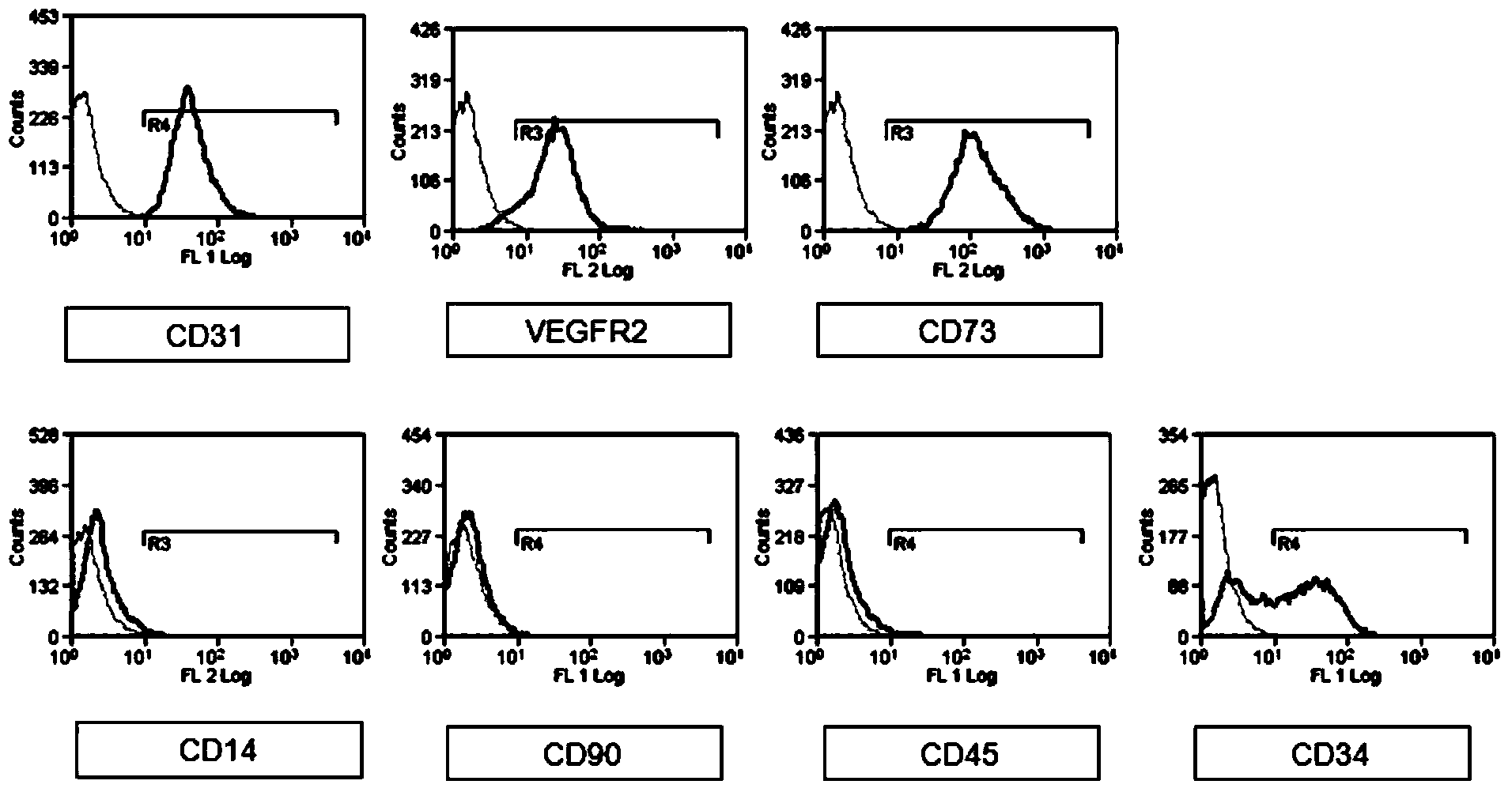
Image
Examples
Embodiment 1
[0024] Example 1 Isolation method of endothelial colony-forming cells of the present invention
[0025] The umbilical cords were collected from healthy puerperas with full-term vaginal delivery or cesarean section. After collection, they were stored in PBS solution containing double antibodies at 4°C and processed within 48 hours. Wash repeatedly, and flush the umbilical cord vein with phosphate-buffered solution (PBS) until the outflow fluid is transparent.
[0026] Clamp one end of the umbilical cord with a hemostat, add type II collagenase (concentration 0.2%) with a pipette gun and fill the umbilical cord vein, and clamp the other end of the umbilical cord with a hemostat; digest on a shaker at 37°C for 30 minutes; after digestion, take Lower the hemostat, collect the digestive juice into a 50ml centrifuge tube; cut the umbilical cord along the umbilical vein to expose the inner surface of the umbilical vein, and gently scrape the umbilical vein with a cell scraper for 5 m...
Embodiment 2
[0028] Example 2 Isolation method of endothelial colony-forming cells of the present invention
[0029] The umbilical cords were collected from healthy puerperas with full-term vaginal delivery or cesarean section. After collection, they were stored in DPBS buffer containing double antibodies at 4°C and processed within 48 hours. Wash repeatedly, and flush the umbilical cord vein with phosphate-buffered solution (PBS) until the outflow fluid is transparent.
[0030] Clamp one end of the umbilical cord with a hemostat, add type II collagenase (concentration 0.2%) with a pipette gun and fill the umbilical cord vein, and clamp the other end of the umbilical cord with a hemostat; sterilize on a shaker at 37°C for 15 minutes; after digestion is complete, take Lower the hemostat, collect the digestive juice into a 50ml centrifuge tube; cut the umbilical cord along the umbilical vein to expose the inner surface of the umbilical vein, and gently scrape the umbilical vein with a cell s...
Embodiment 3
[0032] Example 3 Isolation method of endothelial colony-forming cells of the present invention
[0033] The umbilical cords were collected from healthy puerperas with full-term vaginal delivery or cesarean section. After collection, they were stored in Hanks buffer containing double antibodies at 4°C and processed within 48 hours. Wash repeatedly, and flush the umbilical cord vein with phosphate-buffered solution (PBS) until the outflow fluid is transparent.
[0034] Clamp one end of the umbilical cord with a hemostatic clamp, use a pipette gun to add a mixed enzyme (dual enzyme) composed of type II collagenase (concentration: 0.2%) and trypsin (concentration: 0.5%) at a ratio of 1:1 (v / v) And fill the umbilical cord vein, clamp the other end of the umbilical cord with a hemostatic forceps; 37 ℃ shaker for 60 minutes; after the digestion is completed, remove the hemostatic forceps, collect the digestive juice into a 50ml centrifuge tube; cut the umbilical cord along the umbili...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com