Re-directed immunotherapy
A therapeutic agent, targeted technology, applied in immunoglobulin, anti-animal/human immunoglobulin, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc., can solve the elusive clinical success, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
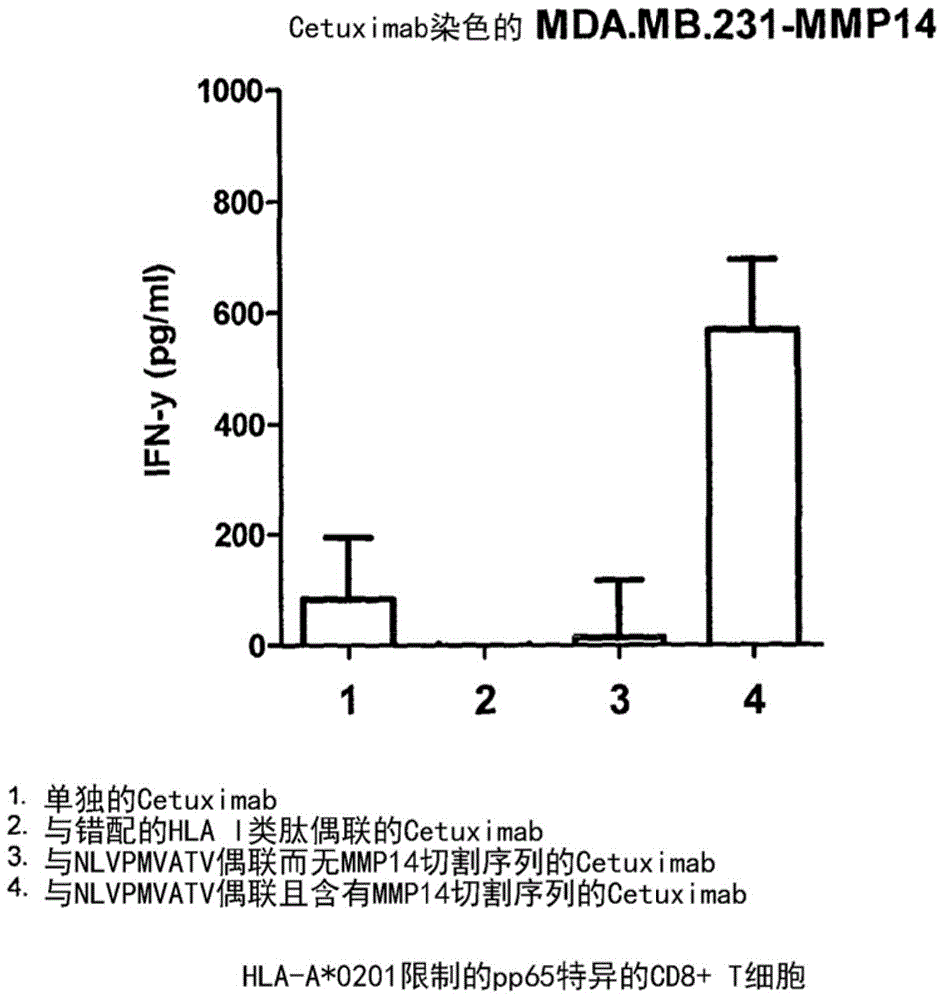
[0282] Example 1: Stimulation of T cells by Cetuximab-NLVMVATV (SEQ ID NO: 21) conjugate
[0283] overview
[0284] We exposed breast cancer cells to an agent containing Cetuximab conjugated to HLA-B7 peptides with and without a cleavage site. Subsequent exposure to T cells led to the generation of a T cell response when the breast cancer cells were exposed to an agent containing the cleavage site.
[0285] result
[0286] MDA.MB.231 cells, which are often used as a model of breast cancer, were transduced with the MMP14 gene to ensure the expression of MMP14 protein in the cells. Unconjugated (1) or coupled with RPHERNGFTVL (SEQ ID No:32), HLA-B7 peptide (2), coupled with NLVPMVATV (SEQ ID No:21) without MMP14 cleavage sequence (3) or with Target cells (1×10 5 ) after staining, the stained cells were incubated overnight. The next day, cells were washed, and NLV-specific T cells were added to the culture (1 × 10 4 ), and incubated overnight. Supernatants were collected...
Embodiment 2
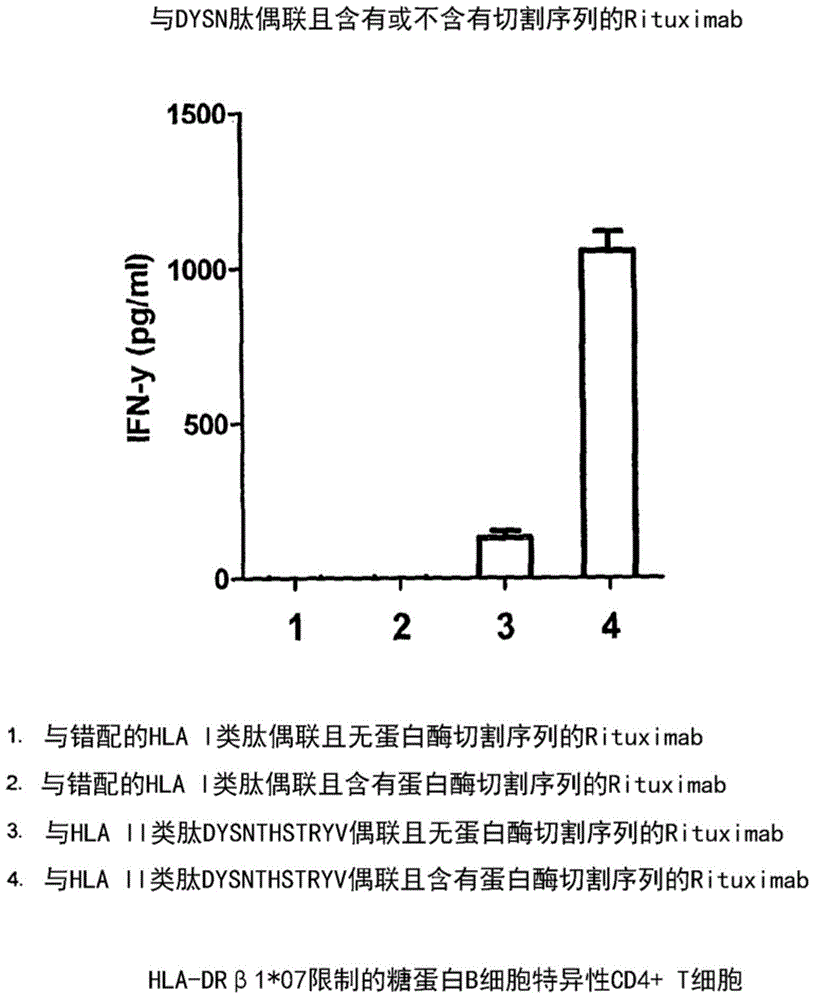
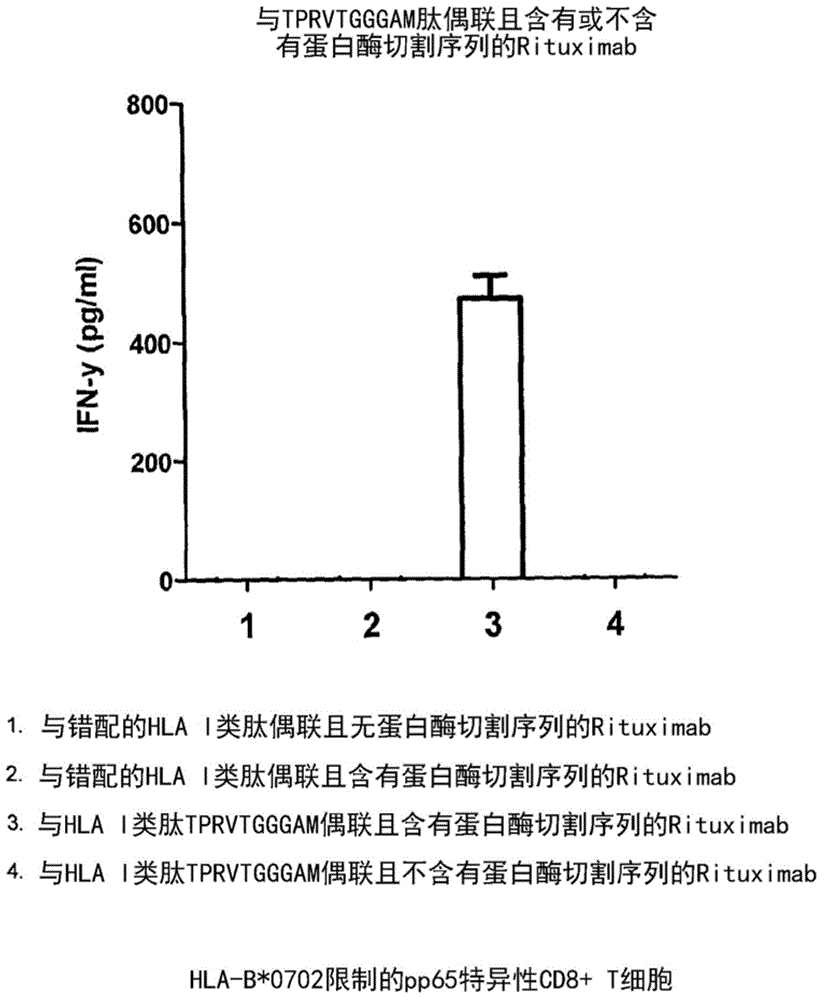
[0288] Example 2: Stimulation of CD4 by Retuximab-DYSNTHSTRYV (SEQ ID NO:55) conjugate + T cells
[0289] overview
[0290] We exposed B-lymphoblastoid cells (B-LCL) to an agent containing Retuximab conjugated to the cytomegalovirus class II HLA-restricted peptide DYSNTHSTRYV (SEQ ID NO:55) with and without a cleavage site. Subsequently, when B-LCL cells are exposed to agents containing cleavage sites, exposure to CD4 + T cells lead to the generation of a T cell response.
[0291] result
[0292] Conjugate unrelated mismatched peptide RPHERNGFTVL (SEQ ID No:32) without protease cleavage sequence, HLA-B7 peptide (1), and irrelevant mismatched class I HLA peptide VLEEETSVML (SEQ ID No:316) with protease cleavage sequence , HLAA-A2 peptide (2), the related peptide DYSNTHSTRYV (SEQ ID No:55) (3) without the protease cleavage sequence or the related peptide DYSNTHSTRYV (SEQ ID No:55) (4) including the protease cleavage sequence, Rituximab will After staining of B-LCL cel...
Embodiment 3
[0294] Example 3: Standard Operating Procedure for Chemically Conjugating Cysteinylated Peptides and Antibodies
[0295] 1. Dissolve cysteinylated peptide in DMSO to a final concentration of 5 mg / ml.
[0296] 2. Weigh 1 mg of sulfosuccinimide 4-[N-maleimidomethyl]cyclohexane-1-carboxylate (thio-SMCC) and dissolve in 500 μl of phosphate-buffered saline (PBS )middle.
[0297] a. Other heterobifunctional cross-linking agents such as thiosuccinimide 6-(3'-[2-pyridyl dithio]-propionamido) hexanoate ( Thio-LC-SPDP ) and N-[β-maleimide propionic acid] hydrazide, trifluoroacetate ( BMHP ).
[0298] 3. Add 20 μl antibody (1 mg / ml, 20 μg antibody) to the dissolved thio-SMCC and incubate at room temperature for 1 hour.
[0299] 4. The protein G column (GE Healthcare) was first washed by centrifuging the column at 13,000 rpm for 30 seconds to remove ethanol (storage buffer).
[0300] 5. Add 500 μl PBS and mix protein G beads well, then centrifuge at 13,000 rpm for 30 seconds. Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com