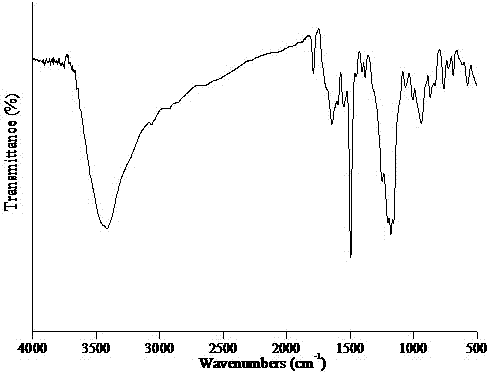
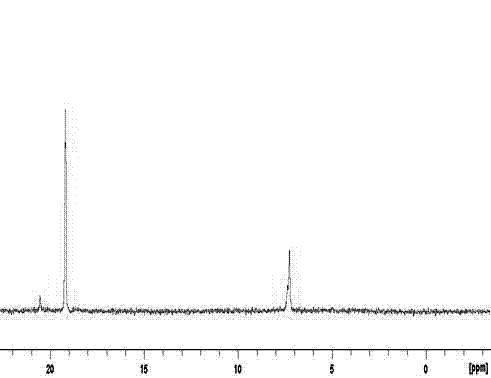
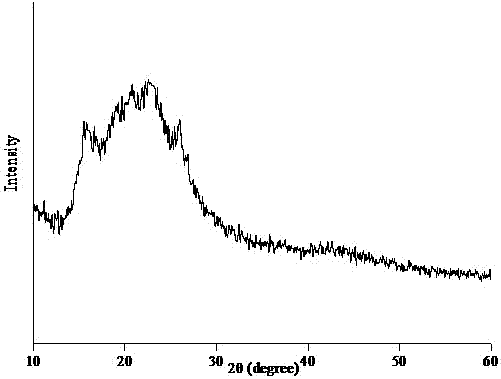
Polycyclotriphosphazene-sulphone phenyl ester and preparation method thereof
A technology of polycyclotriphosphazene and cyclotriphosphazene, which is applied in the field of polycyclotriphosphazene-sulfone phenyl ester and its preparation, can solve the problems of harsh processing and preparation technology, and achieve high temperature solid residual rate and good polarity The effect of solution solubility and high thermal decomposition temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The synthesis of polycyclotriphosphazene-sulfone phenyl ester of the present invention is realized through the following steps:
[0027] (1) In a 100mL three-neck flask equipped with a magnetic rotor stirring, a condenser, and a thermometer, dissolve 6.94g and 0.02mol of hexachlorocyclotriphosphazene prepared by multiple sublimation in 50mL of tetrahydrofuran solvent, and add dropwise 9.28g of the prepared , 0.08mol sodium phenate (under nitrogen protection, 1.84g, 0.08mol metal sodium and 7.52g, 0.08mol phenol reacted in 50mL tetrahydrofuran solution at 25°C for 2 hours) tetrahydrofuran solution, under nitrogen protection, reacted at 40°C for 12 hours . Remove tetrahydrofuran by rotary distillation, wash with deionized water several times, and dry in vacuum at 50°C for 24 hours to obtain a milky white colloid, namely 2,4-dichlorotetraphenoxycyclotriphosphazene; where hexachlorocyclotriphosphazene and phenol The molar ratio of sodium is 1: (3.9~4.1);
[0028] (2) In a...
Embodiment 2
[0032] The synthesis of polycyclotriphosphazene-sulfone phenyl ester of the present invention is realized through the following steps:
[0033] (1) In a 100mL three-neck flask equipped with a magnetic rotor stirring, a condenser, and a thermometer, dissolve 3.47g, 0.01mol of hexachlorocyclotriphosphazene prepared by multiple sublimation in 50mL of tetrahydrofuran solvent, and add dropwise 4.64g of the prepared , 0.04 mol of sodium phenate (under nitrogen protection, 0.92 g, 0.04 mol of metal sodium and 3.76 g, 0.04 mol of phenol were reacted in 50 mL of tetrahydrofuran solution at 25 ° C for 2 hours to prepare a tetrahydrofuran solution, under nitrogen protection, reacted at 40 ° C for 12 hours. Remove tetrahydrofuran by rotary distillation, wash with deionized water several times, and dry in vacuum at 50°C for 24 hours to obtain a milky white colloid, namely 2,4-dichlorotetraphenoxycyclotriphosphazene; where hexachlorocyclotriphosphazene and phenol The molar ratio of sodium i...
Embodiment 3
[0038] The synthesis of polycyclotriphosphazene-sulfone phenyl ester of the present invention is realized through the following steps:
[0039] (1) In a 100mL three-necked flask equipped with a magnetic rotor stirring, a condenser, and a thermometer, dissolve 34.70g and 0.10mol of hexachlorocyclotriphosphazene prepared by multiple sublimation in 50mL of tetrahydrofuran solvent, and add dropwise 46.40g of the prepared , 0.40mol of sodium phenate (prepared by reacting 9.20g, 0.40mol of sodium metal and 37.60g, 0.40mol of phenol in 50mL of tetrahydrofuran solution at 25°C for 2 hours under nitrogen protection) tetrahydrofuran solution, under nitrogen protection, reacted at 40°C for 12 Hour. Remove tetrahydrofuran by rotary distillation, wash with deionized water several times, and dry in vacuum at 50°C for 24 hours to obtain a milky white colloid, namely 2,4-dichlorotetraphenoxycyclotriphosphazene; where hexachlorocyclotriphosphazene and phenol The molar ratio of sodium is 1: (3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com