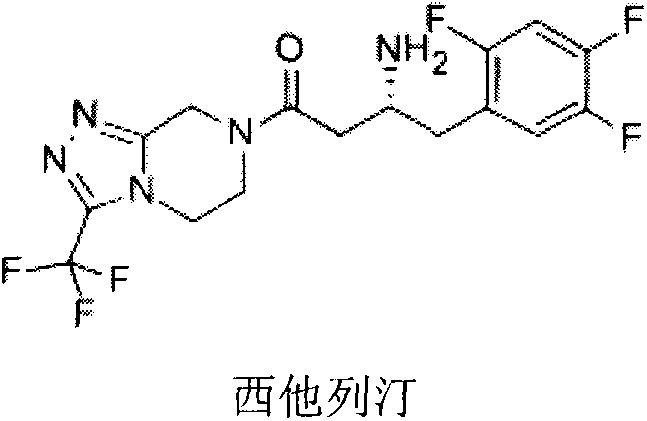
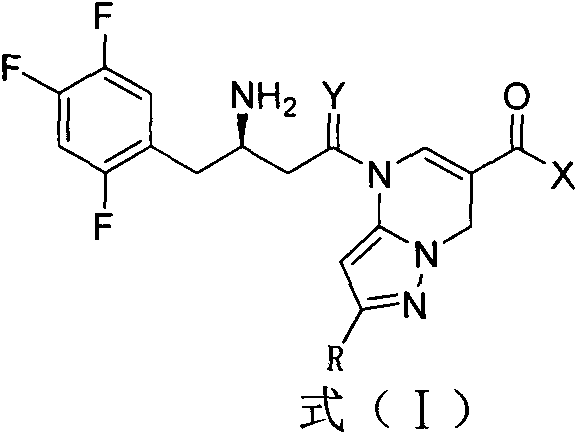
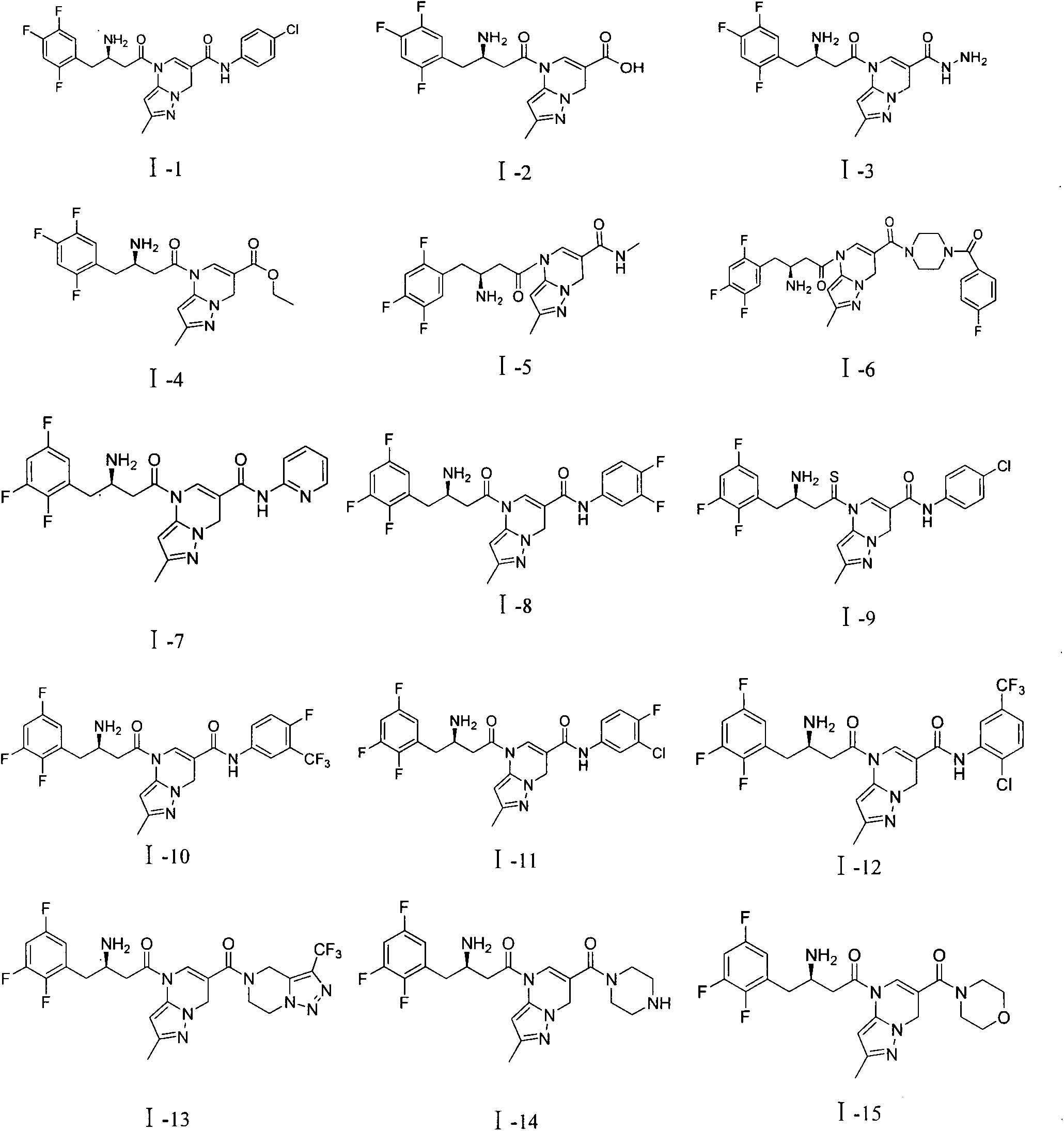
DPP-4 inhibitor with diazine structure
A technology of isomers and compounds, applied in the field of organic synthesis, can solve problems such as inability to meet clinical needs and limited varieties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0074] first step:
[0075]
[0076] Add 8.8g of NaOH and 180ml of methanol to a 250ml single-necked bottle in turn, stir at room temperature to dissolve, add 20.5g of compound (1), heat the oil bath to reflux, TLC (PE:EA=2:1, 2 drops of acetic acid) track the completion of the reaction, Naturally cooled to room temperature, concentrated under reduced pressure to remove the solvent, dissolved the solid in water, adjusted the pH<3 with hydrochloric acid, precipitated a large amount of off-white solid, filtered it with suction, washed the filter cake with water, and dried in vacuo at 45°C to obtain 18 g of a white solid, which is compound (2 ).
[0077] Step two:
[0078]
[0079] Add 0.5g of compound (2), 10ml of DMF, 0.86g of triethylamine, 115g of HOBT and 1.63g of EDC·HCl into a 100ml single-necked flask, stir at room temperature for 1 hour, then add 0.44g of p-chloroaniline, and react overnight at room temperature. TLC (PE:EA=2:1) followed the completion of the re...
Embodiment example 2
[0092] first step:
[0093]
[0094] Add 0.2g of compound (2) to a 500ml single-necked bottle, add 230ml of methanol to dissolve it, then add 0.05g of Pd / C, and after hydrogen replacement for three times, hydrogenate at 35°C under normal pressure. TLC (DCM:MeOH=10:1, adding acetic acid) tracked the completion of the reaction, filtered with suction, and spin-dried the organic phase to obtain 0.18 g of white solid, which was directly used in the next step.
[0095] Step two:
[0096]
[0097] Add 0.56g of compound (5), 20ml of DMF, 0.43g of triethylamine, 0.169g of HOSU and 0.4g of EDC·HCl into a 100ml three-neck flask, stir at room temperature for 1h, add 0.25g of compound (7), and react overnight at 31°C. TLC (DCM:MeOH=40:1, 2 drops of acetic acid) tracked the completion of the reaction, poured the reaction solution into 200ml ice water, a small amount of solid precipitated, adjusted the pH<4 with HCl, extracted three times with EA, combined the organic phases, and wash...
Embodiment 3
[0104] first step:
[0105]
[0106] Add 0.5g of compound (2), 10ml of DMF, 0.86g of triethylamine, 1.15g of HOBT and 1.63g of EDC·HCl into a 100ml single-necked flask, stir at room temperature for 1h, add 0.45g of Boc-hydrazine, and react overnight at 31°C. TLC (PE:EA=1:2, 2 drops of acetic acid) followed the completion of the reaction. Pour the reaction solution into 200ml of ice water to form a yellow solution, extract it three times with EA, combine the organic phases, wash with saturated brine, then dry with anhydrous sodium sulfate for 2 hours, filter with suction, and distill off the solvent under reduced pressure to obtain a red oil 1 g was used directly in the next reaction.
[0107] Step two:
[0108]
[0109] Add 20ml of methanol and 0.1g of Pd / C to a 100ml single-necked bottle containing 1g of compound (10). After three hydrogen replacements, hydrogenation is carried out at normal pressure, and the temperature of the oil bath is controlled at 31°C to react ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com