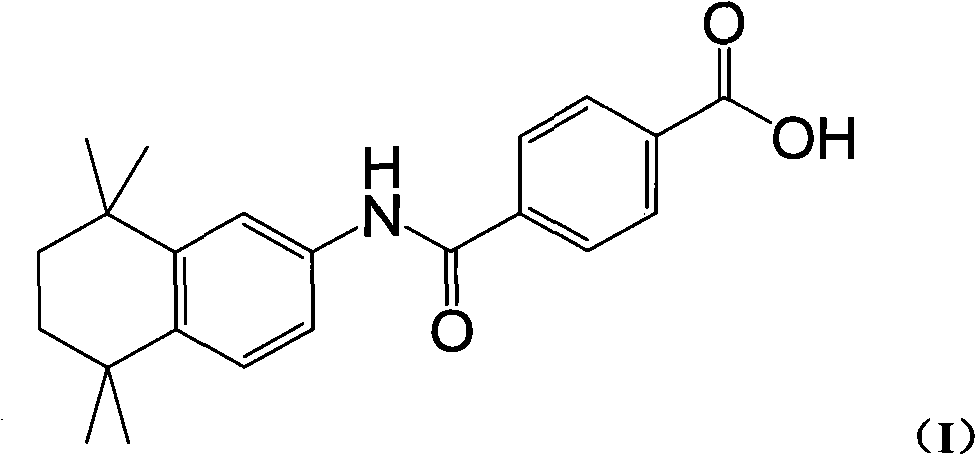
New process for synthesizing tamibarotene
A technology of Tamibarotene and a new method, which is applied in the new process field of Tamibarotene synthesis, can solve the problems of easy generation of by-products, high unit consumption of solvents, and poor solubility of methyl anilbamoylbenzoate And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
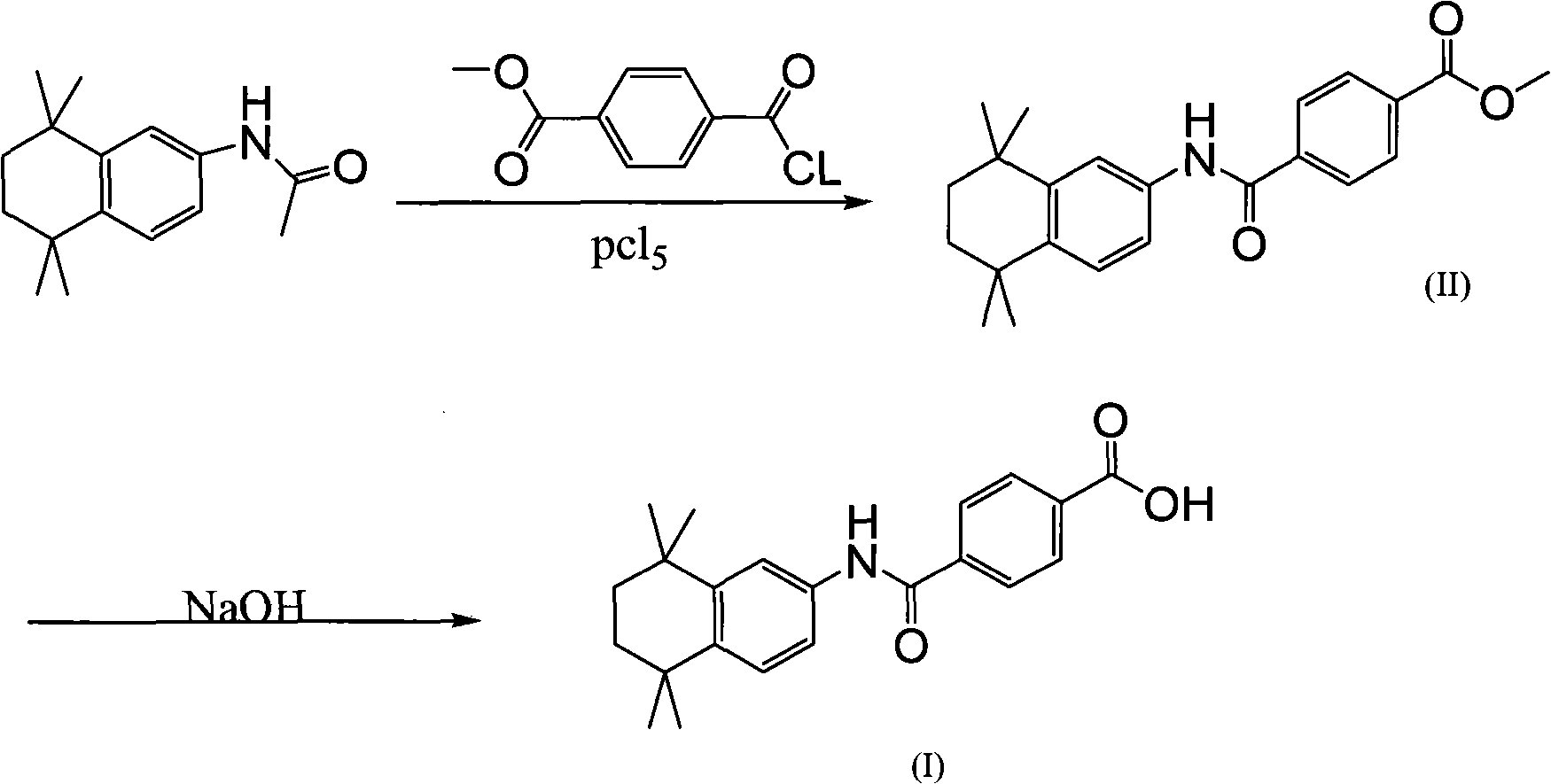
Method used
Image
Examples
example 1
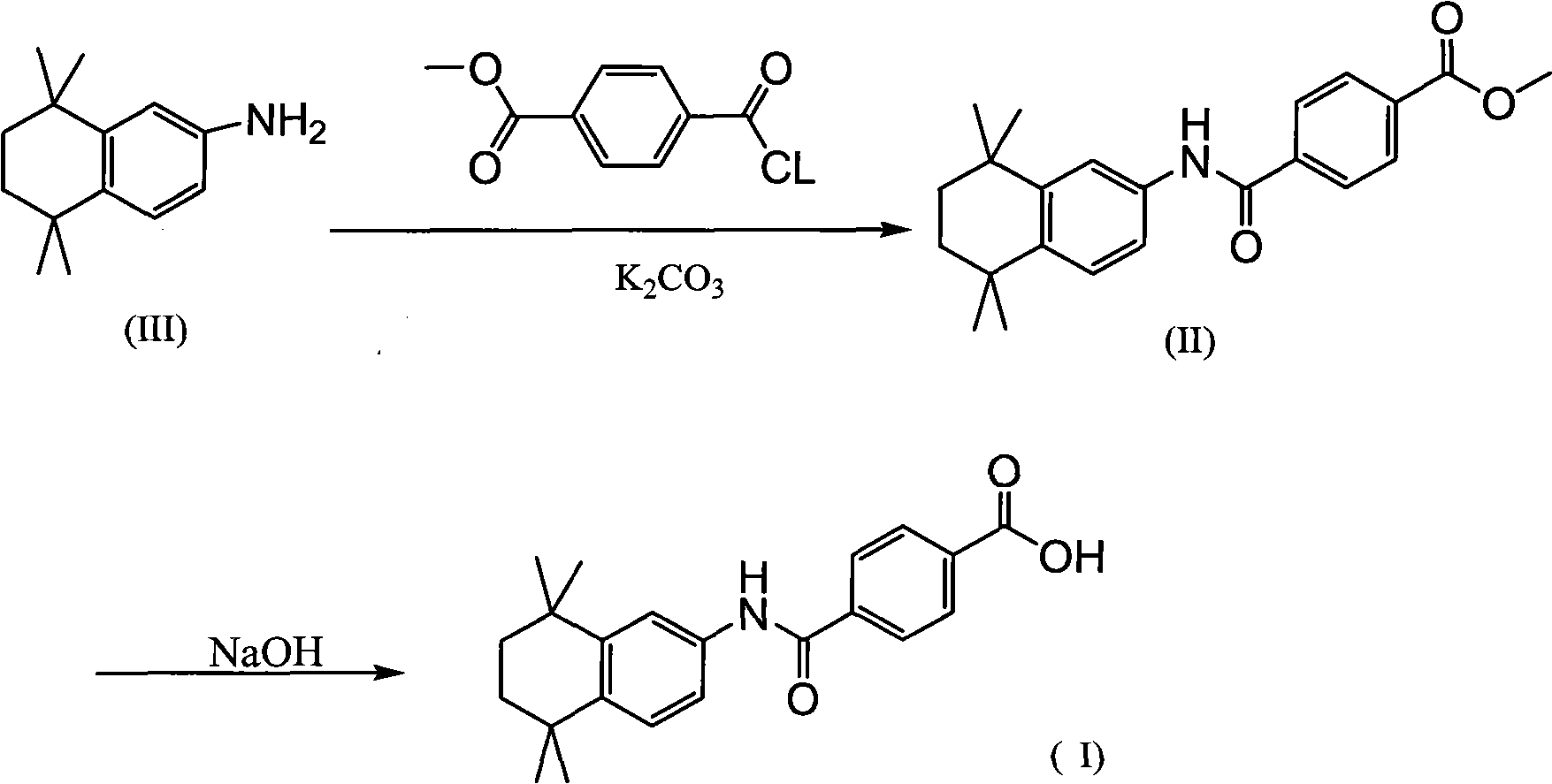
[0025] Preparation of methyl 4-[(4,5,6,7-tetrahydro-5,5,8,8-tetramethyl-2-naphthalene)carbamoyl]benzoate (II)
[0026]
[0027] Dissolve 20.1g of 5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalene-2-amine and 19.4g of monomethyl terephthalate in 200mL of dichloromethane, and then add 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC.HCl) and 33.6 grams each of triethylamine, stirred at room temperature for 24 hours, continued to add 100 mL of hydrochloric acid to the reactant and 500mL of water, separate the water layer, wash once with 500mL of water, once with 300mL of saturated sodium bicarbonate solution, once with saturated saline, dry over anhydrous magnesium sulfate, filter out the desiccant, and distill under reduced pressure to obtain a light yellow solid. Add the solid to 300 mL of ethanol, stir at 50° C. for 12 hours and filter to obtain 29.5 g of white solid.
example 2
[0029] Add 200mL of 10% K in 29.5 grams of compound (II) 2 CO 3 , heated to reflux for 30 minutes, cooled down after the reaction, adjusted the pH to about 2 with hydrochloric acid, and filtered to obtain a white solid. After the solid was dried, it was dissolved in 300 mL of ethanol, and 300 mL of water was slowly added dropwise to precipitate a white solid. After the solid was dried, 25.4 g of Tamibarotene was obtained. The purity of the product was 99.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com