Multivalent antigen-binding FV molecule
A technology of antigen-binding molecules and antigens, applied in the direction of anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, antibody, anti-inflammatory agent, etc., can solve the problem of tandem diabodies lacking the constant domain of immunoglobulin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
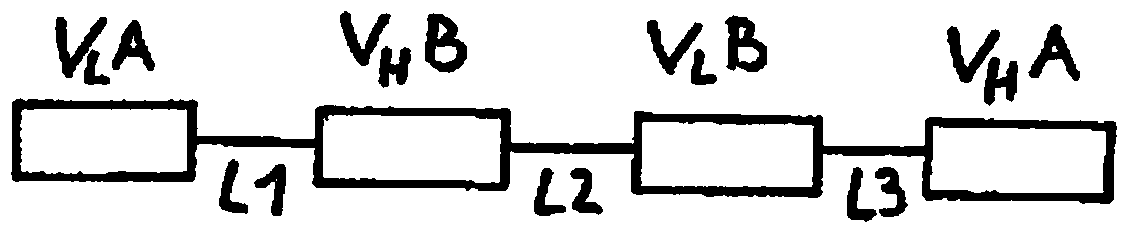
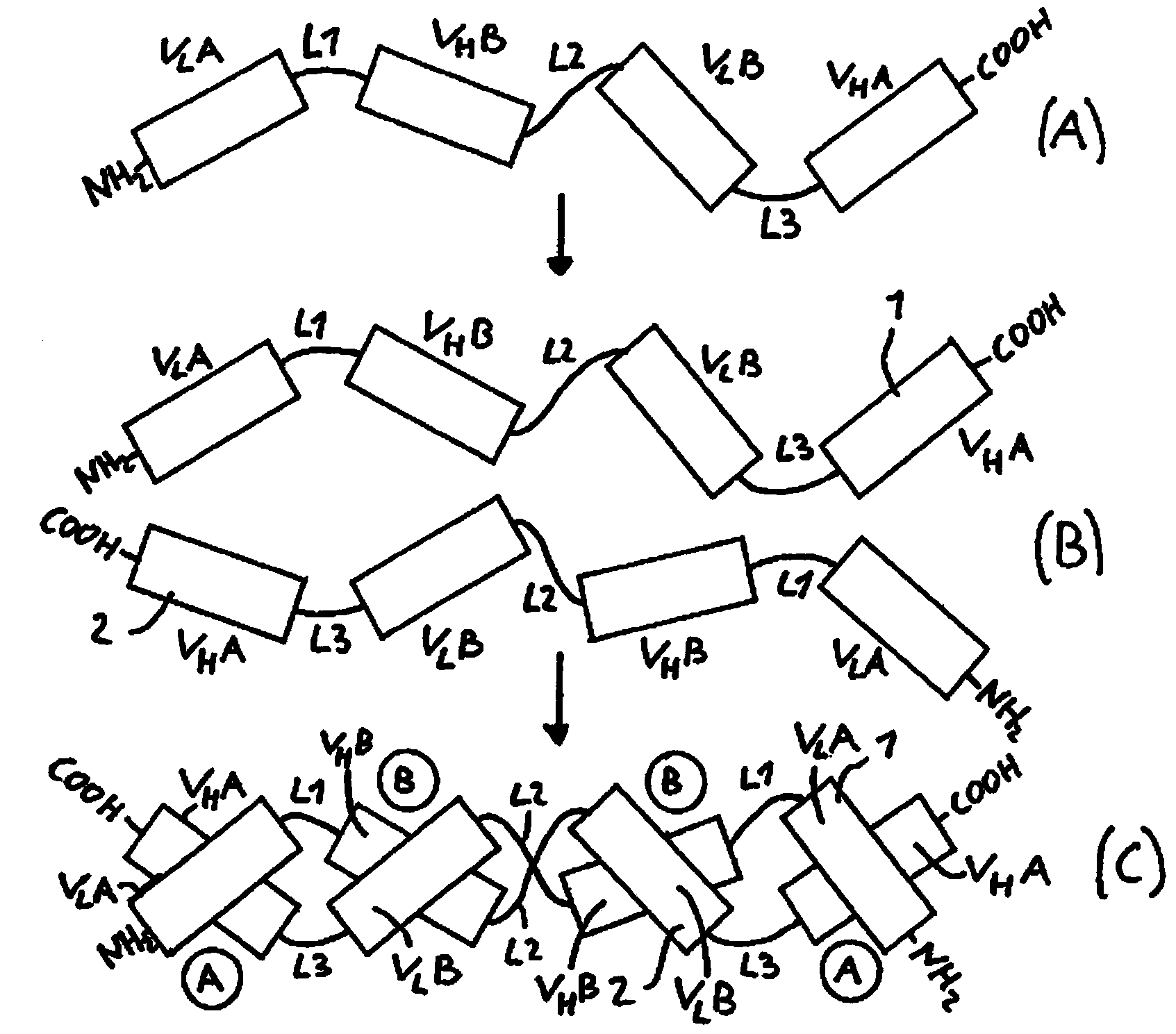
[0090] Divide V for use H A-V L B-V H B-V L The arrangement of the domains outside A constructs a functionalized dimeric tandem diabody ( ), using the two domains of the humanized anti-CD19 single-chain antibody and the humanized anti-CD3 single-chain antibody to use the V of the present invention L A-V H B-V L B-V H The arrangement of the domains of A constructs several such dimeric tandem diabodies. At the same time, the humanized anti-CD39 antibody and the humanized anti-CD3 antibody are subjected to an affinity maturation process, and two variants of each antigen binding molecule are used to confirm the results representing the products of different stages of the affinity maturation process.
[0091] Murine monoclonal antibodies HD37 and UCHT against CD19 and CD3, respectively, are the starting materials for obtaining humanized antibodies with relatively high affinity. In each case, first place V in the scFv phagemid vector H Domain and human V L The library is combined to se...
Embodiment 2
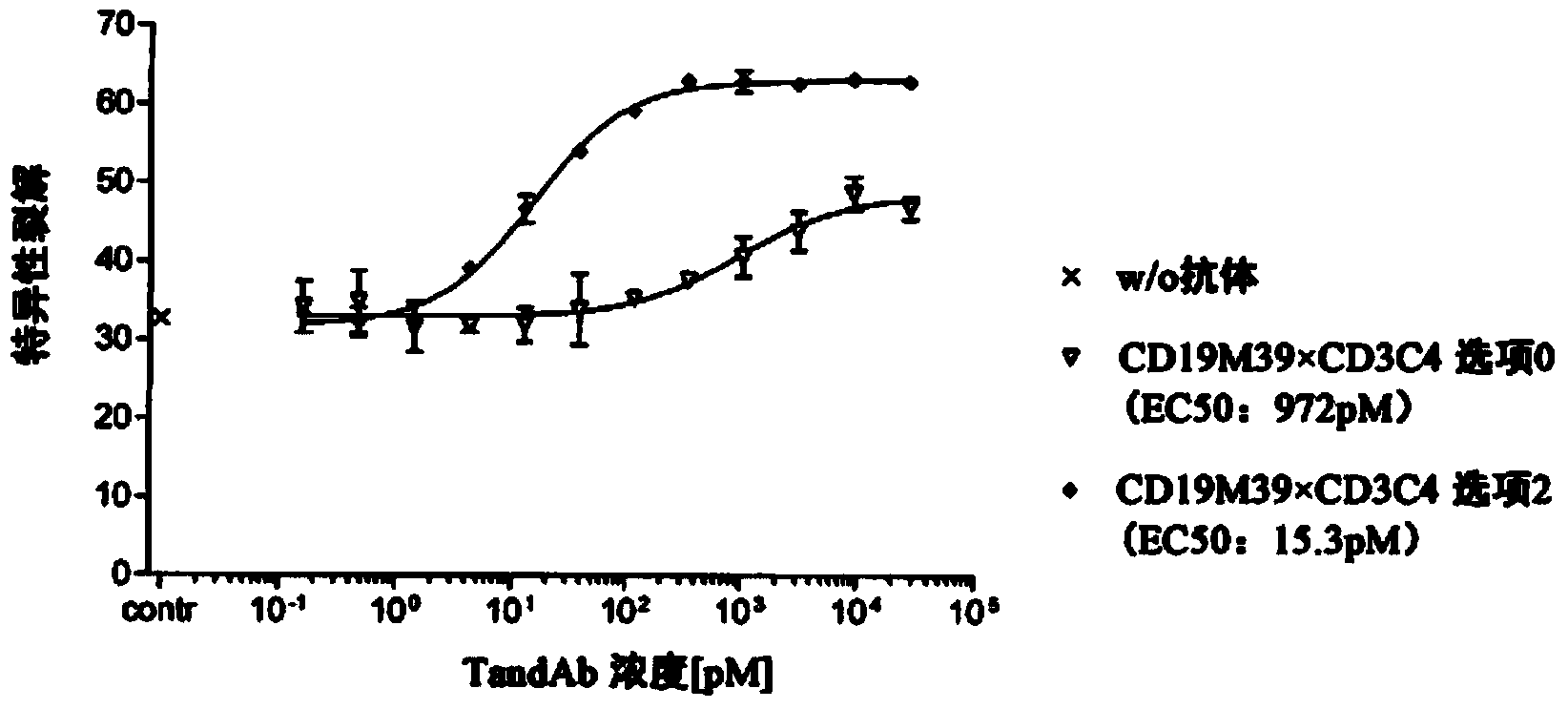
[0119] In vitro T cell receptor regulation by human serum albumin (HSA)×CD3TandAb antibody
[0120] In order to determine whether HSA×CD3TandAb antibodies with different domain sequences have different potency in inducing T cell receptor (TCR) / CD3 regulation in T cells in vitro, cultured in vitro in the presence of increased concentrations of bispecific HSA×CD3TandAb antibodies CD3 + Leukemia cells are then analyzed for the remaining TCR. The conditioning test was performed in the presence or absence of HSA to measure the effect of HSA on TandAb activity.
[0121] In short, 1×10 6 A single leukemia cell was inoculated into each well of a round-bottom 96-well microplate in RPMI1640 medium supplemented with 2mM L-glutamine and 100IU / mL penicillin G sodium salt and 100μg / mL sulfate chain Mycin (all components are from Invitrogen). In different microtiter plates, leukemia cells were seeded in the aforementioned RPMI medium, but 50 mg / mL HSA (Sigma) was added to it. After adding the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com