Flurbiprofen acetaminophen ester solid dispersion and preparation method thereof
A technology of acetaminophen ester and solid dispersion, which is applied in the directions of non-active ingredient medical preparations, active ingredients-containing medical preparations, pharmaceutical formulas, etc., can solve problems such as poor solubility and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
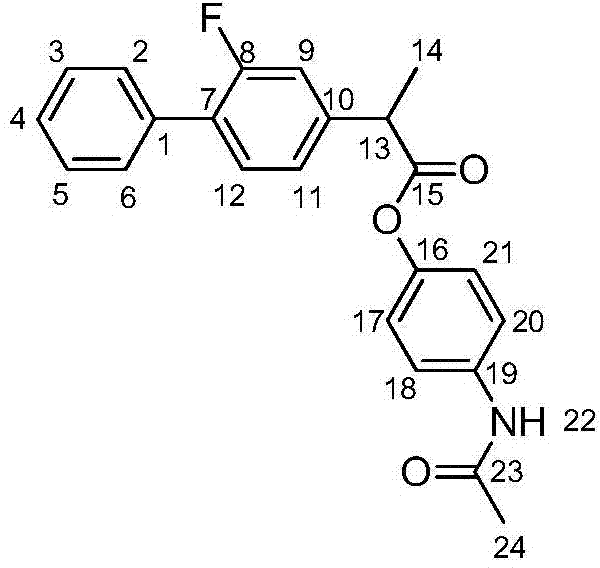
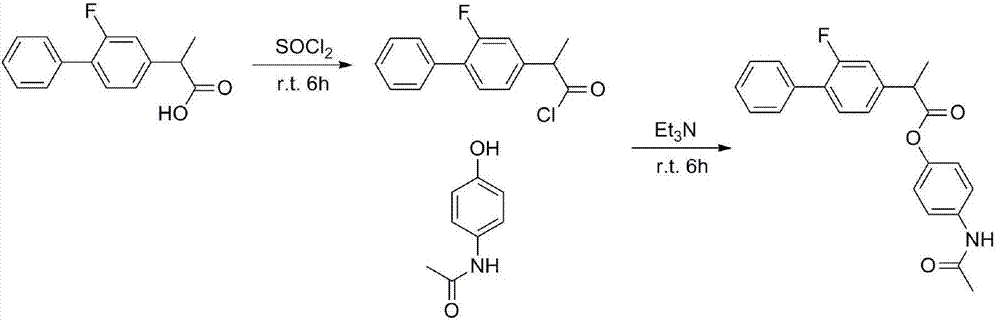
[0033] Embodiment 1: the preparation of flurbiprofen acetaminophen ester
[0034] Weigh 100g (410mmol) flurbiprofen into a round-bottomed flask, add dichloromethane dropwise while stirring until flurbiprofen is completely dissolved in an ice bath, then slowly add the last 45mL (614mmol) dichloromethane Sulfoxide, reacted at room temperature for 6 hours, and evaporated the solvent to obtain 105.5 g of flurbiprofen acyl chloride, with a yield of 98%. Weigh 73g (792mmol) of acetaminophen and place it in a round-bottomed flask, dichloromethane solvent, under ice-bath conditions, slowly add 112mL (803mmol) of triethylamine dropwise, and then the flurbirol dissolved in dichloromethane Fenyl chloride was slowly added dropwise to the acetaminophen solution. Remove the ice bath and react for 6 hours.
[0035] After the reaction, extract with water three times (200mL×3), extract with sodium bicarbonate solution three times (200mL×3), dry the organic phase with anhydrous magnesium sulf...
Embodiment 2
[0043] Take 5g of flurbiprofen acetaminophen ester, dissolve it in ethanol solution, heat PEG400020g to 80°C to form a molten state, slowly add flurbiprofen acetaminophen ester alcohol solution dropwise to the carrier material, stir, and evaporate to dryness Ethanol, quickly moved to -20 ℃ solidified for 4h. The resultant was taken out, pulverized, and dried. That is, solid dispersion powder is obtained. The solid dispersion of the inventive process has an in vitro cumulative dissolution rate of 93.5±3.1% (n=6) within 45 minutes. Six samples were measured in parallel.
Embodiment 3
[0045] Take flurbiprofen acetaminophen ester 5g, dissolve in appropriate ethanol, dissolve PVP-S630 in ethanol, heat at 60°C, mix the above two solutions evenly, stir for 2 hours, evaporate the ethanol, and quickly move to Curing at -20°C for 8 hours. The resultant is taken out, pulverized, and dried to obtain a solid dispersion powder. The solid dispersion of the inventive process has an in vitro cumulative dissolution rate of 94.4±3.6% (n=6) within 45 minutes. Six samples were measured in parallel.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com