Quinazoline derivative and application thereof as vasculogenesis inhibitor
An angiogenesis inhibition and derivative technology, which can be used in drug combination, organic chemistry, anti-tumor drugs, etc., and can solve problems such as drug resistance, adverse reactions, and application limitations.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
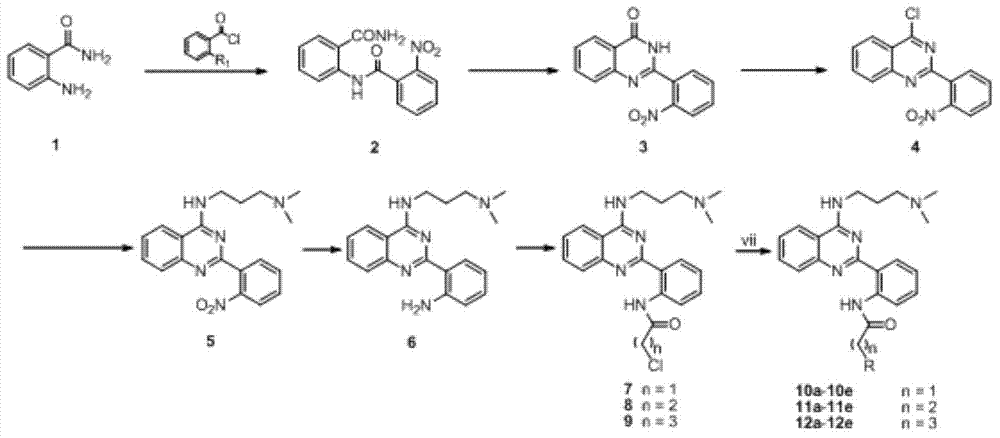
[0019] Synthesis of compound 2
[0020]
[0021] Dissolve 521.6mmol of dry o-nitrobenzoic acid in 60ml of thionyl chloride, evaporate the thionyl chloride after reflux for 2h, and slowly add the obtained brown liquid dropwise to 727.7mmol of anthranilic acid in an ice bath. Formamide and 1455.4 mmol of triethylamine in chloroform (250 ml) were stirred overnight at room temperature, filtered, and washed with ethanol to give 2 as a white solid, yield: 79%. 1 H NMR(400MHz,DMSO)δ12.57(s,1H),8.53(d,J=8.2Hz,1H),8.43(s,1H),8.13(d,J=8.3Hz,1H),7.95–7.78 (m,5H),7.62(dd,J=11.4,4.0Hz,1H),7.29–7.22(m,1H).
Embodiment 2
[0022] Embodiment two: the synthesis of compound 3
[0023]
[0024] After mixing 357.2mmol of dry 2 with 80ml of 10% aqueous sodium hydroxide solution and 80ml of ethanol, they were reacted at 95°C for 6 hours. Ethanol was distilled off, and the pH value of the solution was adjusted to 3 with HCl. A large amount of white solid was precipitated, filtered and dried, and purified by silica gel chromatography (petroleum ether: ethyl acetate = 3:1) to obtain 3 as a white solid with a yield of 91%. 1 HNMR(400MHz,DMSO)δ12.86(s,1H),8.27–8.16(m,2H),7.95–7.81(m,4H),7.67(d,J=7.8Hz,1H),7.62–7.56(m ,1H).
Embodiment 3
[0025] Embodiment three: the synthesis of compound 4
[0026]
[0027] Dissolve 0.0314 mmol of dry 3 in 80 ml of toluene, add 5-10 times molar amount of phosphorus oxychloride and equimolar amount of N,N-diethylaniline, and react under reflux at 105°C for 5 hours. After the reaction, wash with equal volumes of the following solutions in sequence: water, 20% aqueous sodium hydroxide solution (twice), water, saturated saline, 1M hydrochloric acid, and water. The light red flocculent solid precipitated during the washing process was filtered out, filtered, washed with ethanol, and purified by silica gel chromatography (chloroform:methanol=3:1) to obtain white solid 4 with a yield of 74%. 1 H NMR (400MHz,DMSO)δ8.22(ddd,J=16.6,8.0,1.0Hz,2H),7.97–7.82(m,4H),7.69(d,J=7.7Hz,1H),7.63–7.58( m, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com