Application of ginseng saponin IH901 in preparing angiogenesis inhibitor
A technology of angiogenesis inhibition and ginsenosides, which is applied to cardiovascular system diseases, anti-inflammatory agents, organic active ingredients, etc., can solve the problems of unseen ginsenoside IH901, increase myocardial contractility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
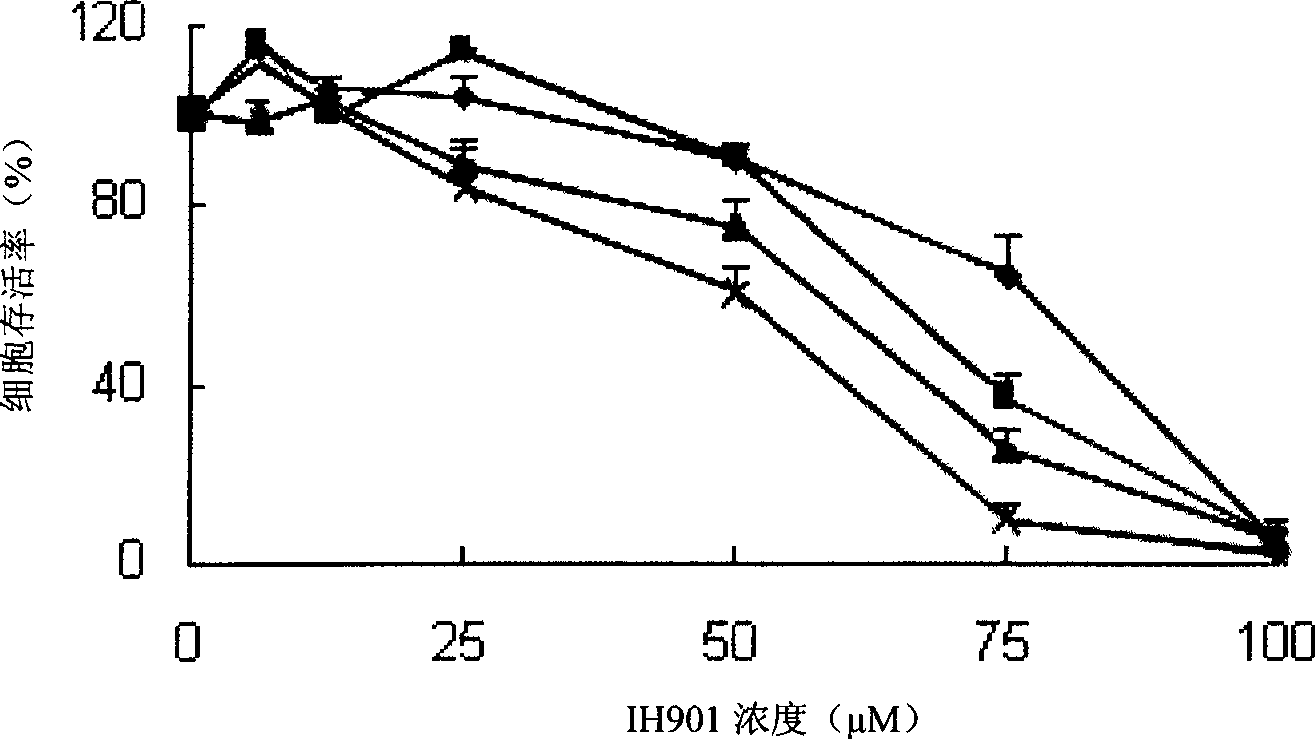
[0029] Embodiment 1 The effect of ginsenoside IH901 on the proliferation of ECV304 cells
[0030] 1) Experimental materials:
[0031] Cell line, culture medium: Human umbilical vein endothelial cells ECV304 were purchased from Shanghai Institute of Cell Biology, Chinese Academy of Sciences. Medium RPMI-1640 was purchased from GIBCO Company, fetal bovine serum was purchased from Hyclone Company, penicillin and streptomycin were purchased from Shanghai Sangong Company.
[0032] 2) Drugs: Ginsenoside IH901 is prepared according to the invention patent method whose publication number is CN1985865; other reagents 3-(4,5-dimethylthiazole-2)-2,5-diphenyltetrazolium bromide (abbreviated as MTT), dimethyl sulfoxide (referred to as DMSO), etc. were purchased from Sigma or other domestic companies.
[0033] 3) Experimental method: A group of ginsenoside IH901 with a concentration of 0, 6.25, 12.5, 25, 50, 75, and 100 μM was applied to ECV304 respectively, and the different concentratio...
Embodiment 2
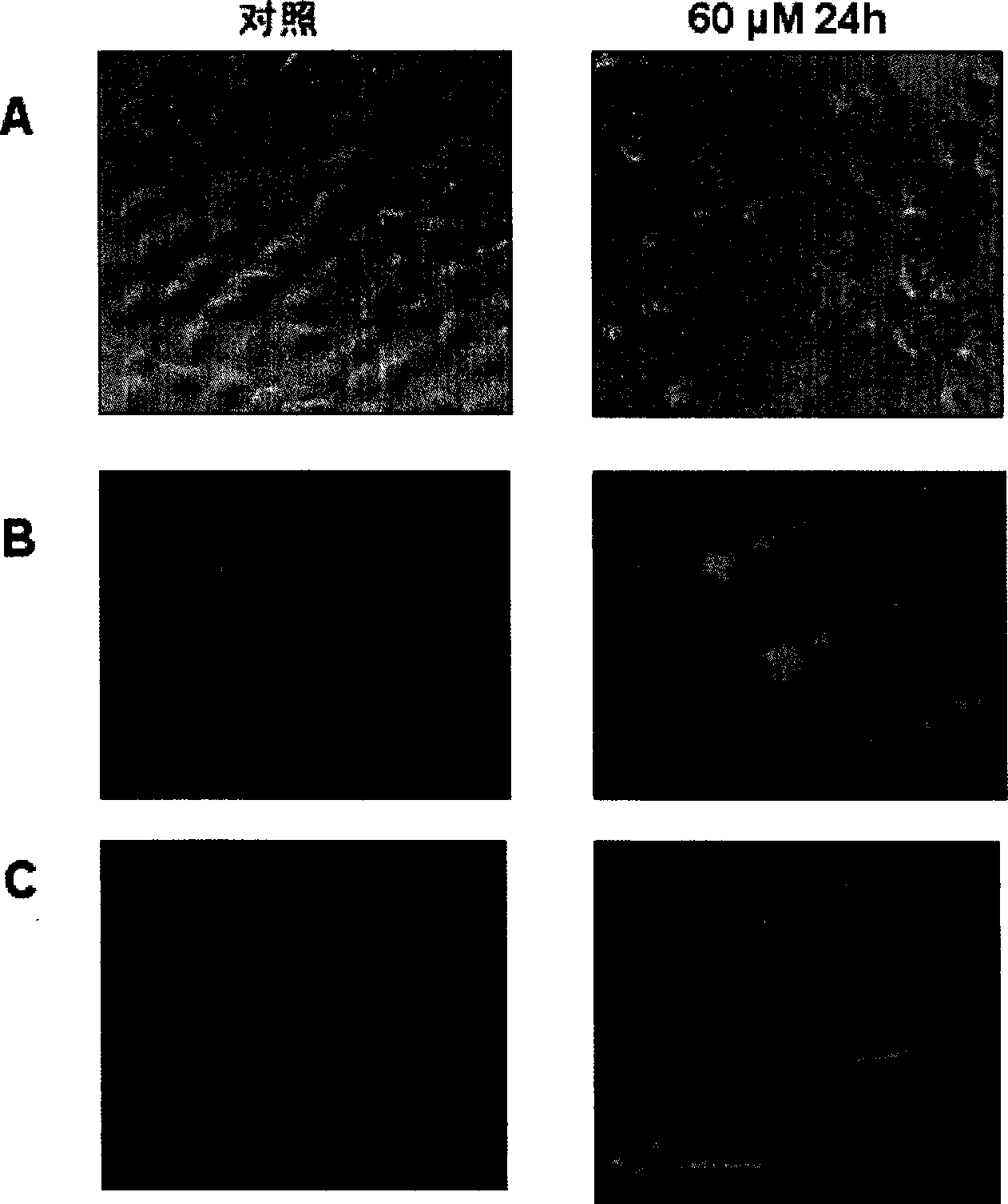
[0038] Example 2 Morphological observation of apoptosis induced by ginsenoside IH901 in ECV304 cells
[0039] 1) Experimental materials: Hoechst 33258, AO / EB were purchased from Calbiochem (San Diego, CA), paraformaldehyde was purchased from Shanghai Sangong Company, and other materials were as in Example 1.
[0040] 2) Experimental method:
[0041] (1) Inoculate the cells in the logarithmic growth phase on the coverslip, take out the slides after drug treatment for a predetermined time, and wash them with PBS for 3 times; (2) Fix with 4% paraformaldehyde for 10 min at room temperature, Wash with PBS for 3 times; (3) Hoechst 33258 staining: After incubating with 2 μl Hoechst 33258 (10 μg / ml) at room temperature for 5-10 minutes, observe under a fluorescent microscope; (4) AO / EB staining: treat treated adherent and After the suspended cells were collected together, they were resuspended with 2 μl of AO (100 μg / ml) and EB (100 μg / ml), observed and photographed under a fluoresce...
Embodiment 3
[0043] Example 3 Effect of ginsenoside IH901 on phase distribution of ECV304 cell cycle
[0044] 1) Experimental materials: Rnase A, Propidium iodide were purchased from Sigma, and other materials were as in Example 1.
[0045] 2) Experimental method:
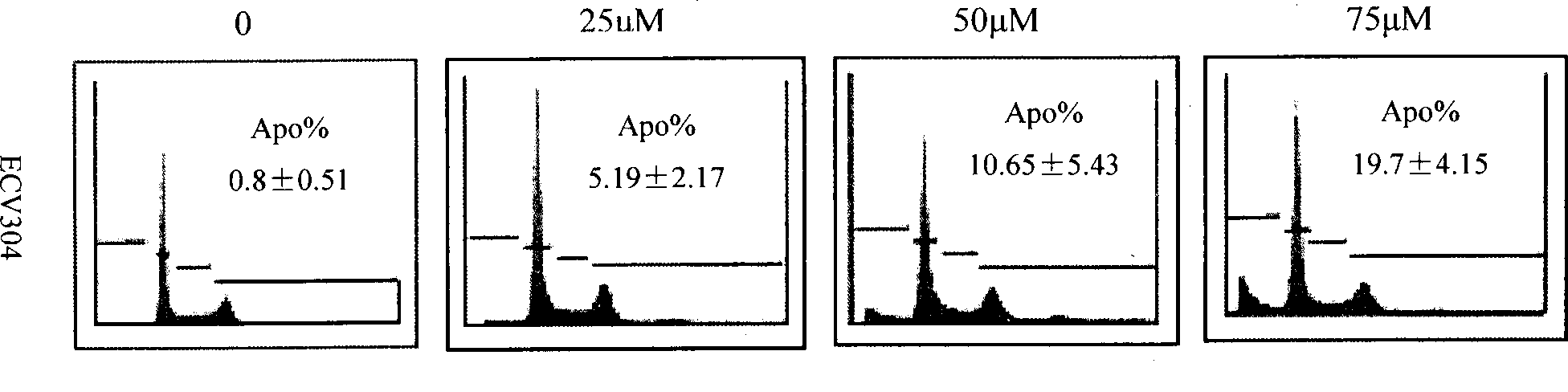
[0046] (1) After the cells in the logarithmic growth phase were treated with ECV304 at 0, 25, 50, and 75 μM IH901 for 12 hours, all adherent and suspended cells were collected; (2) PBS was washed twice and then resuspended in PBS; (3) Fix with pre-cooled 70% ethanol at -20°C for 1 hour, and store at 4°C for testing; (4) remove ethanol after low-speed centrifugation, and wash twice with PBS; Incubate at ℃ for 30 minutes; (6) transfer to an ice bath, add PI (50 mg / ml) dye solution, and incubate at 4 ℃ in the dark for 20-30 minutes; (7) test on the machine.
[0047] 3) Experimental results: cell detection showed that: apoptotic cells increased with the increase of drug concentration, the apoptotic rates were 0.8±0.51%, 5.19±2.17...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com