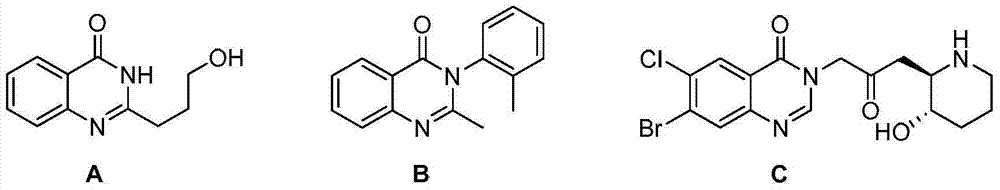
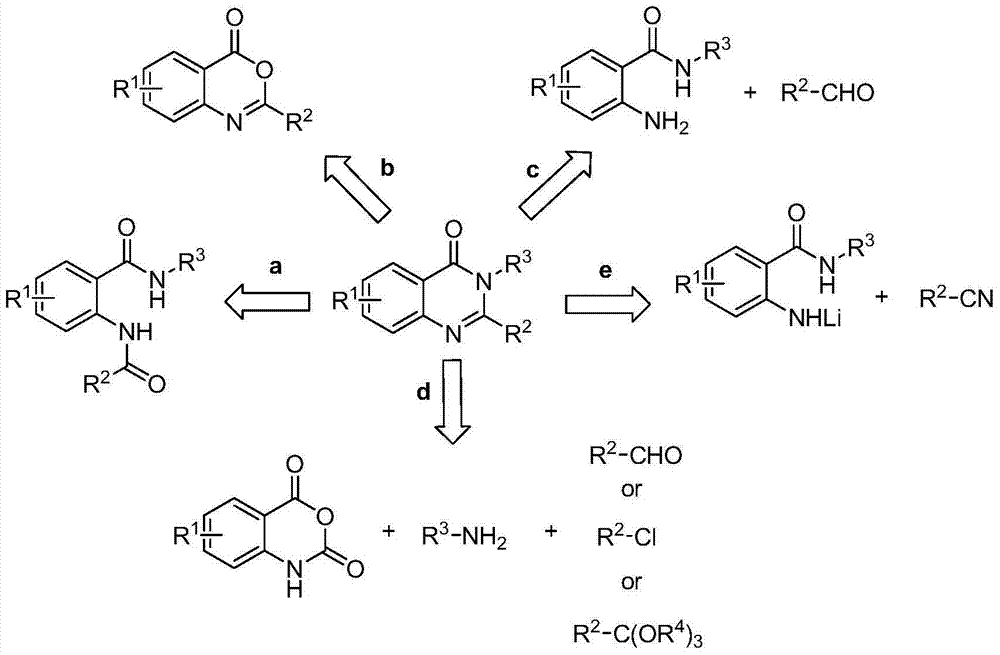
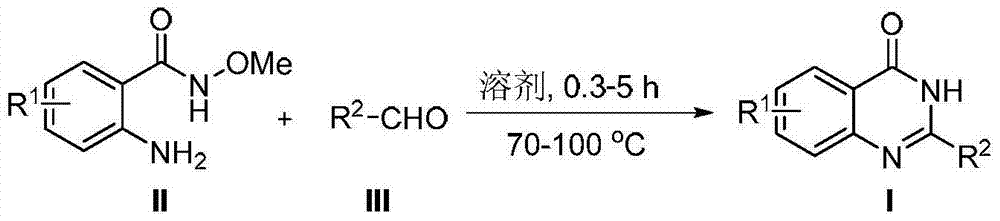
Method for synthesizing 4(3H)-quinazolinone derivative by using cyclization elimination one-pot method
A technology for quinazolinone and derivatives, which is applied in the field of one-pot synthesis of 4(3H)-quinazolone derivatives through cyclization and elimination, which can solve the problems of low product yield, harsh reaction conditions and long reaction time and other problems, to achieve the effect of high yield, simple operation and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A method for synthesizing 4(3H)-quinazolinone derivatives (2-phenylquinazolin-4(3H)-one (I-a)) by one-pot method of cyclization elimination, comprising the following steps:
[0042] 2-Amino-N-methoxybenzamide (II-a) (166 mg) was dissolved in acetic acid (4 ml), and benzaldehyde (III-a) (117 mg) was added thereto, at 100°C The reaction was complete for 1.5 hours, cooled to room temperature, diluted with ethyl acetate (20 ml), then neutralized by adding saturated sodium bicarbonate solution (20 ml), and the aqueous phase was extracted with ethyl acetate (3 × 20 ml ), the combined organic phases were washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure to obtain a crude product. The crude product was separated and purified by silica gel column chromatography to obtain 206 mg of 2-phenylquinazolin-4(3H)-one (I-a) as a white solid with a yield of 93%.
[0043] 1 H NMR (600MHz, DMSO-d 6):δ12.56(br s,1H),8.21(d...
Embodiment 2
[0045] A method for synthesizing 4(3H)-quinazolinone derivatives (2-phenylquinazolin-4(3H)-one (I-a)) by one-pot method of cyclization elimination, comprising the following steps:
[0046] 2-Amino-N-methoxybenzamide (II-a) (166 mg) was dissolved in formic acid (4 ml), and benzaldehyde (III-a) (117 mg) was added thereto, at 100°C The reaction was complete under 5.0 hours, cooled to room temperature, diluted with ethyl acetate (20 ml), then neutralized by adding saturated sodium bicarbonate solution (20 ml), the aqueous phase was extracted with ethyl acetate (3 × 20 ml ), the combined organic phases were washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure to obtain a crude product. The crude product was separated and purified by silica gel column chromatography to obtain 89 mg of 2-phenylquinazolin-4(3H)-one (I-a) as a white solid with a yield of 40%.
[0047] 1 H NMR (600MHz, DMSO-d 6 ):δ12.56(br s,1H),8.21(d,J...
Embodiment 3
[0049] A method for the one-pot synthesis of 4(3H)-quinazolinone derivatives (2-(4-bromophenyl)quinazolin-4(3H)-one (I-b)) by cyclization elimination, comprising the following steps step:
[0050] 2-Amino-N-methoxybenzamide (II-a) (166 mg) was dissolved in trifluoroacetic acid (4 ml), to which was added p-bromobenzaldehyde (III-b) (204 mg) , reacted at 70°C for 1.0 hour to complete the reaction, cooled to room temperature, added ethyl acetate (20 ml) to it for dilution, then added saturated sodium bicarbonate solution (20 ml) for neutralization, and extracted the aqueous phase with ethyl acetate ( 3×20 ml), the combined organic phases were washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure to obtain a crude product. The crude product was separated and purified by silica gel column chromatography to obtain 245 mg of 2-(4-bromophenyl)quinazolin-4(3H)-one (I-b) as a white solid with a yield of 82%.
[0051] 1 H ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap